|
Case Report
Cesarean scar pregnancy mimicking a pseudo-gestational sac: A case report with magnetic resonance imaging findings
1 MD, Resident, Gynecology and Obstetrics, Chibune General Hospital, Osaka City, Osaka, Japan
2 MD, PhD, Attending, Gynecology and Obstetrics, Chibune General Hospital, Osaka City, Osaka, Japan
3 MD, Attending, Radiology, Chibune General Hospital, Osaka City, Osaka, Japan
4 MD, Fellow, Gynecology and Obstetrics, Chibune General Hospital, Osaka City, Osaka, Japan
Address correspondence to:
Hitomi Futaki
3-29-3, Fuki-machi, Nishiyodogawa-ku, Osaka City, Osaka 555-0034,
Japan
Message to Corresponding Author
Article ID: 100028G06HF2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Futaki H, Oki N, Maeda T, Kashima Y, Inagaki M, Yoshida S. Cesarean scar pregnancy mimicking a pseudo-gestational sac: A case report with magnetic resonance imaging findings. Edorium J Gynecol Obstet 2022;7(2):5–9.ABSTRACT
Introduction: A corpus luteal cyst may reportedly be misidentified as an ectopic gestational sac. We report a case of cesarean scar pregnancy mimicking a pseudo-gestational sac, to differentiate between the two entities and diagnose correctly.
Case Report: A 42-year-old woman with four previous cesarean sections, at 7 weeks of gestation, was suspected of an unknown site pregnancy. Transvaginal Doppler ultrasonography showed a small cystic structure accompanied by neither a yolk sac nor surrounding marginal flow, adjacent to the uterine scar, and a 16-mm-large low-echo area with a white ring in the left adnexa. Pelvic magnetic resonance imaging also demonstrated a small cystic structure without contrast enhancement resembling a pseudo-gestational sac adjacent to the cesarean scar. Moreover, a cystic structure with ring-enhancement beside the left ovary, which mimicked an ectopic gestational sac, was also detected. Given these imaging findings and slightly elevated serum β-human chorionic gonadotropin (β-hCG) levels, ectopic pregnancy in the left fallopian tube could not be ruled out. She underwent laparoscopic surgery and uterine content removal, which resulted in intrauterine miscarriage. The cystic structure partially resected from a marginal area in the ovary appeared to be a luteal cyst.
Conclusion: For correct diagnosis and appropriate treatment, the clinical circumstances should be fully considered without excessive reliance on imaging findings.
Keywords: Cesarean scar pregnancy, Ectopic pregnancy, Gestational sac, Miscarriage
INTRODUCTION
Ectopic pregnancy (EP) should be carefully managed because it can result in maternal death. The incidence of EP has also increased with the advancement in assisted-reproductive technology, occurring in approximately 1.5–2.1% of patients undergoing in vitro fertilization [1],[2],[3]. Cesarean scar pregnancy is an extremely rare type of EP: there is the implantation of the gestational sac onto the anterior wall of the uterus at the site of at least one previous cesarean section. Due to the rise in cesarean delivery rates, the incidence of cesarean scar pregnancies has steadily increased over the years [4]. Joshi et al. [5] described the case of a previous cesarean section delivery in a woman with a viable gestational sac in the lower uterine segment and elevated β-human chorionic gonadotropin (β-hCG) levels. They indicated that the possibility of a scar ectopic pregnancy should be considered. However, diagnosis can be difficult in cases where these clinical findings are inconclusive. Herein, we report a case of cesarean scar pregnancy in which it was difficult to make a correct diagnosis due to several confusing clinical and imaging findings.
CASE REPORT
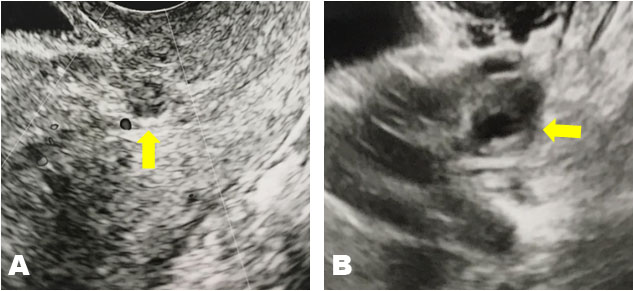
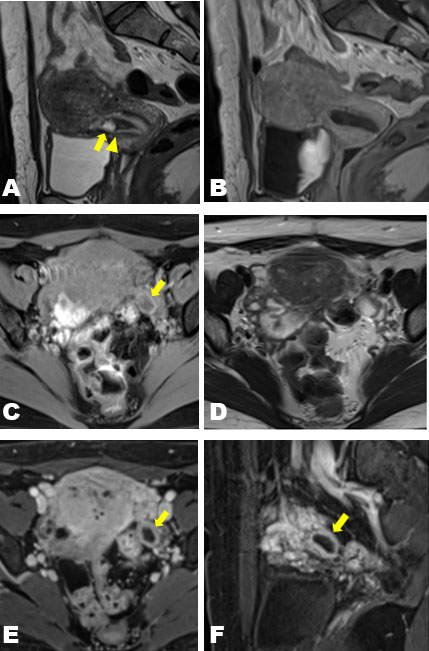
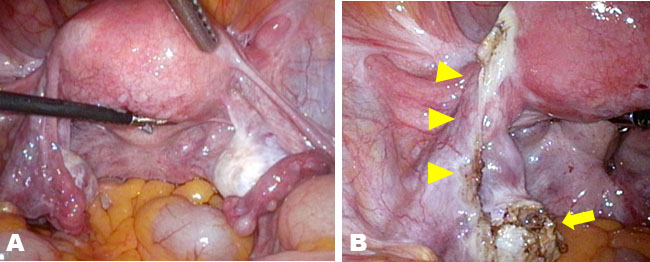
A 42-year-old woman at 7 weeks of gestation in her sixth pregnancy was referred to our clinic on the suspicion of a pregnancy at an unknown location. She had a history of four cesarean deliveries and one spontaneous abortion. The patient’s medical history was unremarkable. At presentation, her vital signs were within normal limits and stable. Physical examination was notable only for minor genital bleeding. The patient’s hemoglobin and hematocrit levels were within normal limits. The β-hCG level was 2194 IU/L. Transvaginal ultrasonography revealed a 10-mm-large cystic structure without a yolk sac and blood flow adjacent to the scar of the uterus (Figure 1A) and a 16-mm-large low-echo area accompanied by a white ring in the left adnexa (Figure 1B). Pelvic magnetic resonance imaging (MRI) demonstrated a cystic structure lacking contrast enhancement on the lower uterine segment adjacent to the cesarean scar while demonstrating a cystic structure with ring enhancement beside the left ovary (Figure 2). Because of the clinical suspicion of left-tube EP, the patient underwent diagnostic and therapeutic laparoscopy and endometrial curettage. Both ovaries were normal (Figure 3A), and a structure that appeared to be a luteal cyst was found in the left ovary, which was partially resected (Figure 3B). Surgical management included left salpingectomy, wedge dissection of the ovary, and endometrial curettage. Chorionic villi were macroscopically found in the intrauterine specimens, and histological findings confirmed an early abortion in the uterus. The postoperative course was uneventful. The β-hCG level decreased to 61 IU/L on postoperative day 4, confirming normalization on an outpatient basis.
DISCUSSION
The commonest site of EP is the fallopian tube, accounting for 96% of all EPs [6]. Other sites of EP include the ovary, abdomen, myometrium, cervix, and previous cesarean scar. In some cases, the site of pregnancy cannot be determined even after close examination. Cesarean scar pregnancy is one of the rarest types of EPs, occurring in 1 in 2000 pregnancies [7]. However, with the increase in cesarean sections in recent years, cesarean scar pregnancies have also risen substantially. The diagnostic criteria for EP in the scar are as follows: (1) an empty uterine cavity and cervical canal, (2) a gestational sac in the anterior part of the uterine isthmus, (3) the absence of healthy myometrium between the bladder and gestational sac, (4) and the circular blood flow surrounding the sac must be clearly visible [8],[9],[10]. In the present case, transvaginal ultrasonography identified a sac-like structure without marginal blood flow in the anterior part of the lower uterine segment with an empty uterine cavity and a low echo area accompanied by a white ring in the left adnexa. Magnetic resonance imaging also revealed similar findings. However, the findings of this case were inconsistent with the above criteria; an adnexal structure mimicking a gestational sac and slightly elevated serum β-hCG levels were also noted. This explains why we diagnosed the uterine sac-like structure as a pseudo-gestational sac. However, we could not rule out EP of the fallopian tube, and we eventually performed laparoscopic surgery.
The current sac-like structure on the cesarean scar was not proven to have vascularity with either ultrasonography or MRI, hence mimicking a pseudo-gestational sac. Thus, the diagnosis of scar EP was not made confidently, partly because of its rarity. In addition, the adnexal cystic structure with hypervascularity resembled a gestational sac, which further confirmed the diagnosis. Given that tubal EP frequently appears as a thick-walled cystic lesion in the adnexa, care must be taken to differentiate normal corpus luteum cysts from EP [11]. To definitively differentiate a corpus luteum cyst from a tubal EP, it must be demonstrated that the thick-walled cystic lesion originates from the ovary because EPs in the ovary are exceedingly rare [12]. In addition, another distinguishing factor is that corpus luteum cysts typically demonstrate high T1 signal intensity in their walls [13]. It was difficult to identify whether the current adnexal cystic structure, with a thick wall and hypervascularity, was in or out of the ovary. However, it demonstrated a high T1 signal intensity in the wall on MRI, which could have been the key to a correct diagnosis.
Trends in β-hCG are instrumental to diagnosis of pregnancy. With early normal progressing intrauterine pregnancies (IUPs), β-hCG levels increase by at least 53% within 48 h. With a failing IUP, the β-hCG levels decline by 21–35% within 48 h [14]. Ectopic pregnancy is suspected in pregnancies without expected increases or decreases in β-hCG levels. No single pattern characterizes EPs, and approximately half of the EP would show decreasing β-hCG levels, whereas the other half would show an increase [15]. In the initial examination of the present case, β-hCG was 2194 mIU/mL, and it declined by only 17% within 48 h. This mild decrease in β-hCG levels supports the diagnosis of EP in the fallopian tube. The process of diagnosis and treatment of EP remains controversial. While the most common treatment for fallopian tube pregnancy is surgery because of its curative and immediate effects [16], some institutions recommend that dilation and curettage could be performed in all cases except for those that have ruptured to avoid extra laparoscopic surgery because guessing the site of pregnancy by β-hCG, transvaginal ultrasonography, or MRI may sometimes be incorrect. In this case, the most suspicious pregnancy site was the left adnexa. Therefore, the patient underwent laparoscopic surgery first. However, since there was also the possibility of a cesarean scar area pregnancy, we should have considered performing dilation and curettage first to avoid additional laparoscopic surgery. In contrast, dilation and curettage carry the risk of intrauterine adhesions and uterine perforation, particularly in patients who have undergone multiple cesarean sections, as in this case. Therefore, it may have been reasonable to perform dilation and curettage in the surgical theater instead of a treatment room.
CONCLUSION
In patients with EP, to diagnose correctly and perform an appropriate treatment, full consideration of the clinical circumstances and assessment of changes over time is important without excessive reliance on imaging findings. In addition, if the risk of uterine perforation is high, as in this case, performing dilation and curettage in a surgical theater is preferable.
REFERENCES
1.
Londra L, Moreau C, Strobino D, Garcia J, Zacur H, Zhao Y. Ectopic pregnancy after in vitro fertilization: Differences between fresh and frozen-thawed cycles. Fertil Steril 2015;104(1):110–8. [CrossRef]
[Pubmed]

2.
Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol 2006;107(3):595–604. [CrossRef]
[Pubmed]

3.
Yoder N, Tal R, Martin JR. Abdominal ectopic pregnancy after in vitro fertilization and single embryo transfer: A case report and systematic review. Reprod Biol Endocrinol 2016;14(1):1–69. [CrossRef]
[Pubmed]

4.
Chukus A, Tirada N, Restrepo R, Reddy NI. Uncommon implantation sites of ectopic pregnancy: Thinking beyond the complex adnexal mass. Radiographics 2015;35(3):946–59. [CrossRef]
[Pubmed]

5.
Joshi SD, Momin SA, Shetty D. An unusual case of live caesarean scar ectopic pregnancy: A common entity in an uncommon location. Pol J Radiol 2017;82:296–8. [CrossRef]
[Pubmed]

6.
Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: A 10 year population-based study of 1800 cases. Hum Reprod 2002;17(12):3224–30. [CrossRef]
[Pubmed]

7.
Jayaram PM, Okunoye GO, Konje J. Caesarean scar ectopic pregnancy: Diagnostic challenges and management options. Obstet Gynecol 2017;19(1):13–20. [CrossRef]

8.
Roy MM, Radfar F. Management of a viable cesarean scar pregnancy: A case report. Oman Med J 2017;32(2):161–6. [CrossRef]
[Pubmed]

9.
Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol 2003;21(3):220–7. [CrossRef]
[Pubmed]

10.
Saiyyed A. Ruptured caesarean scar ectopic pregnancy. Int J Reprod Contracept Obstet Gynecol 2018;7(3):1249–50. [CrossRef]

11.
Parker RA 3rd, Yano M, Tai AW, Friedman M, Narra VR, Menias CO. MR imaging findings of ectopic pregnancy: A pictorial review. Radiographics 2012;32(5):1445–60. [CrossRef]
[Pubmed]

12.
Grimes HG, Nosal RA, Gallagher JC. Ovarian pregnancy: A series of 24 cases. Obstet Gynecol 1983;61(2):174–80.
[Pubmed]

13.
Tamai K, Koyama T, Togashi K. MR features of ectopic pregnancy. Eur Radiol 2007;17(12):3236–46. [CrossRef]
[Pubmed]

14.
Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol 2004;104(1):50–5. [CrossRef]
[Pubmed]

15.
Barnhart K, Sammel MD, Chung K, Zhou L, Hummel AC, Guo W. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: Defining the normal curve. Obstet Gynecol 2004;104(5 Pt 1):975–81. [CrossRef]
[Pubmed]

16.
Hsu JY, Chen L, Gumer AR, et al. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol 2017;217(1):49.e1–49.e10. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
We would like to thank Editage (https://www.editage.com) for English language editing.
Author ContributionsHitomi Futaki - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Noriyoshi Oki - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Tetsuo Maeda - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yoko Kashima - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mieko Inagaki - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Shigeki Yoshida - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Hitomi Futaki et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.