|
Case Series
Outcomes of pregnancy in patients with Hodgkin lymphoma
1 Haematology Unit, Medical Department, Hospital Tengku Ampuan Afzan, Kuantan, Malaysia
2 Maternal Fetal Medicine Unit, Obstetrics & Gynaecology Department, Hospital Tengku Ampuan Afzan, Kuantan, Malaysia
3 Pathology Department, Hospital Tengku Ampuan Afzan, Kuantan, Malaysia
Address correspondence to:
Sopian Abdul Wahab
Haematology Unit, Hospital Tengku Ampuan Afzan, Jalan Tanah Putih, 25100, Kuantan,
Malaysia
Message to Corresponding Author
Article ID: 100025G06SW2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Wahab SA, Husin R, Salleh H, Kori AN. Outcomes of pregnancy in patients with Hodgkin lymphoma. Edorium J Gynecol Obstet 2022;7:100025G06SW2022.ABSTRACT
Introduction: Hodgkin lymphoma (HL) is a common lymphoma that occurs in young age group. Thus, the occurrence of HL is inevitable. However, as the disease implies, there is no single study to prove the safety use of chemotherapy in pregnancy.
Case Series: As for our center, in 2019 we encountered three patients with diagnosis of HL which was diagnosed at 16 weeks, 30 weeks, and 32 weeks of gestation. The outcome of the pregnancy was 1 death due to severe prematurity, and two live births. The details of patient presentation, histology, imaging for staging, progress and outcome of pregnancy were reviewed via the patient’s medical record.
Conclusion: The outcomes of pregnancies may varies depending on the timing of the diagnosis and the exposure of chemotherapy in the stage of pregnancy.
Keywords: Chemotherapy, Hodgkin lymphoma, Pregnancy, Prematurity
INTRODUCTION
Hodgkin lymphoma (HL) is a common lymphoid neoplasm that affects women in reproductive age. The incidence of the disease is estimated around 1 in 1000 and 1 in 3000 of pregnancies [1]. There is limited data in comparing the effectiveness of different treatment regimens and these treatment approaches are limited to the case reports or case series as well as expert opinion. The majority of authors suggested to avoid an alkylating agent and to best preserve the normal fetal development with minimal maternal compromised [2].
As for our center, in 2019 we encountered three patients with diagnosis of HL which was diagnosed at 16 weeks, 30 weeks, and 32 weeks of gestation. The outcome of the pregnancy was 1 death due to severe prematurity, and two live births. The details of patient presentation, histology, imaging for staging, progress and outcome of pregnancy were reviewed via the patient’s medical record.
Case Series
Case 1
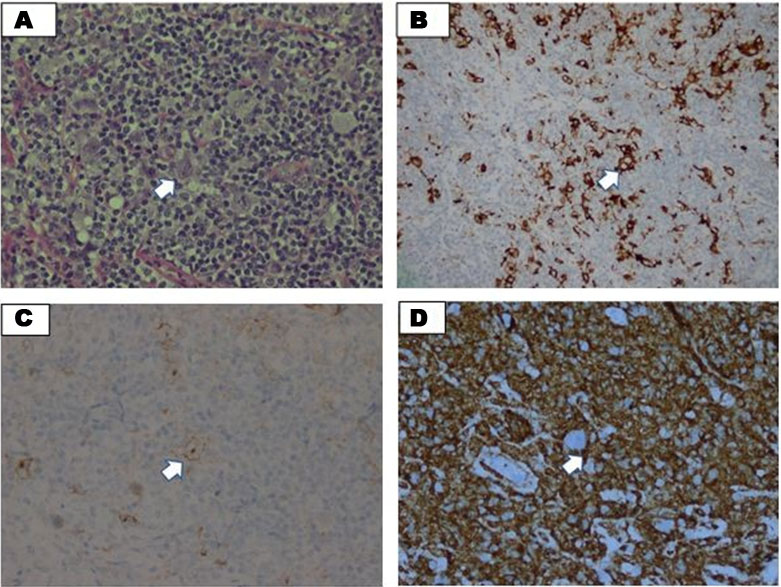
Madam HI is a 26-year-old woman Para 2 with only 1 living child and a history of severe premature delivery complicated with neonatal death during the second trimester. She was diagnosed with nodular sclerosing HL, stage II bulky disease, primary cervical at 16 weeks of pregnancy. She was presented to Hospital Segamat (one of the District Hospitals in Peninsular Malaysia) for bilateral neck swelling which was increasing in size for the past five months associated with B symptoms. Her full blood count at the presentation showed hemoglobin (Hb) of 10.4 g/dL, platelet of 432 × 109/L, and the total white blood cell (TWBC) of 10.5 × 109/L, her lactate dehydrogenase (LDH) was 350 mmol/L, and her erythrocyte sedimentation rate (ESR) of 50 mm/h, based on histopathological examination (HPE) of her lymph node biopsy revealed a diffuse discohesive sheet of neoplastic cells and mixed with rich mature lymphocytes and some plasma cells. Neutrophils and eosinophils are rarely seen. Some mummified cells are seen showing condensed cytoplasm with pyknotic nuclei. No necrosis or fibrosis seen. The Ki67 proliferative index is around 60%. Immunohistochemistry showed positivity towards CD15 & CD30, CD3 cells are seen rosetting around the Reed-Sternberg cells. The overall features were suggestive of lymphocyte-rich classical HL (Figure 1A, Figure 1B, Figure 1C, Figure 1D). She started on the first cycle of adriamycin, bleomycin, vinblastine and dacarbazine (ABVD; doxorubicin of 25 mg/m2, bleomycin of 10 mg/m2, vinblastine of 6 mg/m2, and dacarbazine of 375 mg/m2) at 17 weeks of pregnancy. She was planned to be delivered at 36 weeks of pregnancy. However, she started to have contraction pain at 28 weeks of pregnancy and delivered spontaneously via vaginal delivery. At that point, she has completed 3 cycles of ABVD. Her baby weighed 1.0 kg with a poor Apgar score of 4/10 at 1 minute thus needing intubation and Neonatal Intensive Care Unit (NICU) admission. The baby succumbed on day 3 of life due to severe prematurity.
Case 2
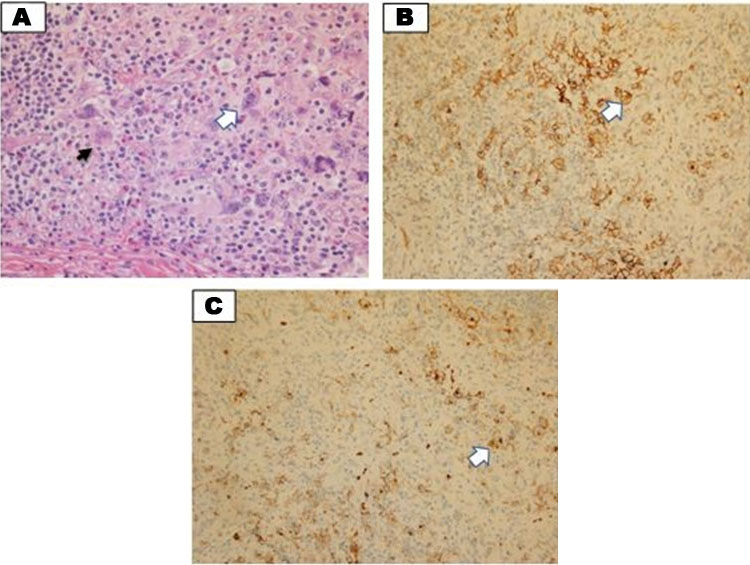
Madam NA is a 25-year-old woman primigravida, who was diagnosed with nodular sclerosing HL, stage II, bulky disease, primary mediastinal at 32 weeks of pregnancy. She was initially presented with abdominal pain and was treated as pyelonephritis, clinical examination revealed multiple cervical lymphadenopathies, however no hepatosplenomegaly of note. Her blood parameters at diagnosis were as follows; Hb of 8.3 g/dL (mean corpuscular volume, MCV: 69 fL; mean corpuscular hemoglobin, MCH: 22 PG), platelet of 266 × 109/L and TWBC of 7.76 × 109/L, her LDH was 250 mmol/L, and her ESR was 70 mm/h. Her iron study showed serum iron of 4 μmol/L, transferrin saturation of 20% and serum ferritin of 200 μg/L. Her HPE of lymph nodes showed effacement of nodal architecture by a few cellular nodules surrounded by collagen bands. The nodules are composed of neoplastic lymphoid cells in a background of numerous lymphocytes with eosinophils and neutrophils. Many lacunar Hodgkin/Reed-Stenberg cells are seen. Immunohistochemistry (IHC) showed CD30 and CD15 positive cells suggestive of nodular sclerosis classical HL (Figure 2A, Figure 2B, Figure 2C). She started with the ABVD regimen at 32 weeks of pregnancy and was delivered at 36 weeks of pregnancy after receiving 1 cycle of ABVD, she was also supplemented with iron sucrose infusion with a total of 1000 mg over a period of 2 weeks and her hemoglobin picked up to 10.5 g/dL after completion. The baby was delivered via emergency lower segment caesarian section (EMLCS) due to fetal distress. The baby weighed 2.7 kg, with a good Apgar score at 1 and 5 minutes which was 8 and 9, respectively. She has oligohydramnios with an amniotic fluid index (AFI) of 6.5 of note with no evidence of fetal anemia.
Case 3
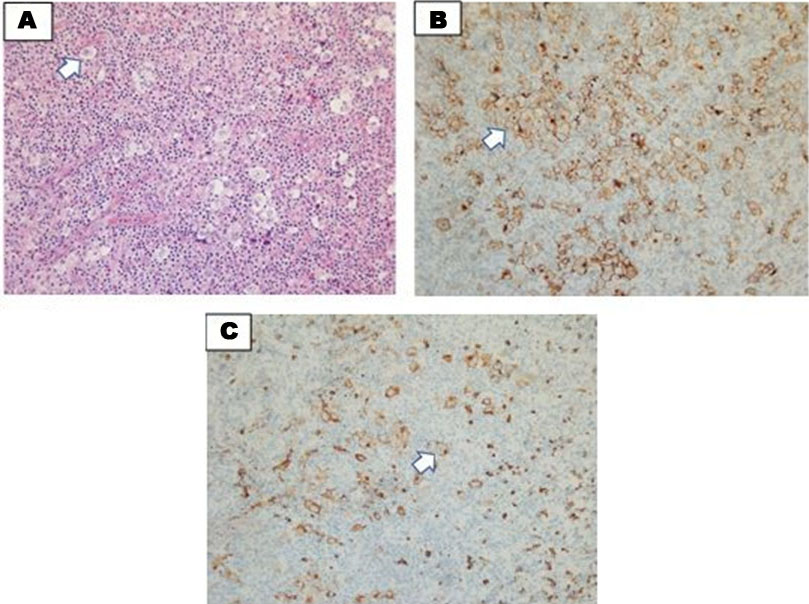
Madam NS is a 27-year-old woman Para 3, who initially presented when she was 30 weeks pregnant with multiple cervical lymphadenopathies for two months. She was diagnosed with nodular sclerosing HL stage II disease. Her baseline blood parameters showed Hb of 9.5 g/dL (MCV: 65, MCH: 22), platelet of 447 × 109/L and TWBC of 4.2 × 109/L. Her LDH and ESR were 215 mmol/L and 52 mm/h, respectively. Her HPE of cervical lymph nodes showed architectural distortion with a nodular growth pattern exhibiting multiple nodules surrounded by fibrous collagen bands. Aggregates and singly disperses large atypical neoplastic cells were seen with non-neoplastic inflammatory background. These neoplastic cells exhibited large monolobated cells with irregular nuclear membrane, mummified cells, bilobated to multilobated as well as lacunae cells. The inflammatory background was composed of eosinophils, lymphocytes, and plasma cells. The IHC study showed the neoplastic cells were positive towards CD30, CD15 with Golgi accentuation and dim positivity for PAX5. The overall HPE and IHC findings were consistent with nodular sclerosis, classical HL (Figure 3A, Figure 3B, Figure 3C). She was started on ABVD regimen and delivered at 36 weeks of pregnancy after 3 cycles of chemotherapy. Her baby weighed 2.4 kg with a good Apgar score at 1 minute and 5 minute which were 8 and 9, respectively. Amniotic fluid index was reduced toward the end of pregnancy thus she was induced for delivery.
DISCUSSION
Hodgkin lymphoma is the common hematological malignancy that originated from the germinal center of lymph nodes. It is among the most curable diseases even though it has an aggressive course of progression. Hodgkin lymphoma is estimated to account for about 10% of cases of newly diagnosed lymphoma in the United States (8260 of 80,500) [3]. Based on WHO 2016 classification of tumors of hematopoietic and lymphoid tissues, HL has been classified into two main subtypes, namely classical HL (CHL) consisting more than 90% of all HL and nodular lymphocyte predominant HL (NLPHL) which encompass less than 5% of all HL cases [4]. The CHL is further divided into four subtypes, namely nodular sclerosis, unclassified, mixed cellularity as well as lymphocyte depleted subtype [5].
The diagnostic test for HL in pregnancy needs to be done as to determine the morphological appearance with complete IHC assessment. Even though fine needle aspiration cytology (FNAC) study might be the easiest way with minimal complication; however, the sample is almost always inadequate to assess the architecture as well as the IHC assessment [6]. The core needle biopsy might be the preferred choice as the diagnostic yield is almost up to 90% with complete assessment and no repeat biopsy is needed as compared to FNAC which can be diagnosed only 10% of lymphoma cases with higher rate for the needs of repeat biopsy [7]. Other than core needle biopsy, the excisional or incisional biopsy is the other diagnostic tools that can be used as well. The other biochemical parameters need to be assessed as well as part of the baseline assessment which includes complete blood count, lactate dehydrogenase, erythrocytes sedimentation rate as well as liver and renal function profile [2].
In terms of disease staging and treatment response assessment in pregnancy, even though the combined F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) is the preferred modality, but this should not be advocated in the case of a pregnant woman [8]. Thus, limited but informative assessment is to be done with as lower side effect as possible toward the mother and fetus. The assessment includes a proper chest radiograph with abdominal shield combined with abdominal ultrasonography might be the preferred choice to assess the abdominal nodes involvement. Other than that, magnetic resonance imaging (MRI) without gadolinium might be the alternative if source is not an issue [2].
The treatment of choice for HL depending on the risk stratification at presentation whether it is an early stage, intermediate stage, or advanced stage. The German Hodgkin Study group (GHSG) advocated the use of ABVD in early and intermediate stage disease and escalated BEACOPP uses higher dosage of cyclophosphamide, doxorubicin, and etoposide in advanced stage disease [9]. The cure rate for early/intermediate stage and advanced stage disease is 90% and 70%, respectively [9]. However, the choice of treatment regimens in HL during pregnancy is limited. The treatment approach is much dependent on the trimester of pregnancy whether it is diagnosed in the first trimester or second or third trimester.
There are no single guidelines to suggest the best treatment of choice during the pregnancy; however, most of the authors recommended a multidisciplinary approach involving Obstetrician, Hematologist, and Radiologist in providing the best care of pregnant women with HL. An extensive and holistic discussion in regard to treatment options, safety and efficacy, outcome of pregnancy, and patient’s wish need to be addressed and discussed. As far as the treatment option is concerned, many reports suggest avoiding the first trimester for chemotherapy administration; however if the disease is in advance stage, symptomatic and/or organ compromised, termination of pregnancy might be the best option. If the disease occurred in the second or third trimester, depending on the disease status whether it is an early or advanced stage disease, single agent vinblastine until delivery or ABVD if the disease progress may be instituted [2].
During the first trimester, where the organogenesis mainly occurs, the chemotherapy used during this period of pregnancy may affect the fetus development especially the cardiac, limbs, neural tube, ears, and eyes [10]. The administration of cytotoxic agents during this period is associated with high risk of malformations, embryo death, and spontaneous abortion. The risk of malformations is estimated around 7–17% when a single agent is used and increases to 25% in cases of combination therapy [11]. Thus, most experts suggested to delay the initiation of chemotherapy until after the end of first trimester of pregnancy. It is estimated around 1.3% of newborn with mother receiving chemotherapy during the first trimester to develop major malformations [12]. Moreover, there is several studies to suggest the use of chemotherapy during second and third trimester of pregnancy has minimal side effect to the outcome of the child [13].
The decision of timing of delivery is mainly depending on the fetal maturation. As far as the pregnancy age is concerned, the best is to delay the delivery until the favorable fetal maturation is achieved. As much as possible, the delivery should be delayed between 35 and 37 weeks of pregnancy [2]. The commonest outcome in pregnant women treated with chemotherapy during the second and third trimester of pregnancy is preterm deliveries with almost 54% of the cases [10]. In cases mentioned above, we notice that both were successfully delivered at 36 weeks with the indications were mainly obstetrics and no fetal abnormality observed. The incidence of small-for-gestational age was observed in 24.2% of patients receiving chemotherapy in pregnancy; however, in our cases the main complication was oligohydramnios [10].
The decision for delivery needs to be discussed with neonatologist especially if the baby needs to be delivered preterm. The neonatologist should be readily available to assess the baby postpartumly and the decision for NICU care needs to be done as soon as possible to avoid complications. With regard to the breastfeeding, it should be avoided as most of the cytotoxic agents are secreted in the breast milk. The assessment of the disease needs to be done postpartumly which includes PET/CT scan to look for the disease status. The chemotherapy regimens need to be continued to complete full course of 6 to 8 cycles and if the patient received only received vinblastine during the pregnancy the full dose of chemotherapy regimens should be started from the beginning with PET/CT assessment to be done prior [2].
CONCLUSION
In general, HL is a common hematological malignancy manifested during pregnancy and it is best managed by multidisciplinary team to provide the best treatment option thus reducing the maternal and fetal complications.
REFERENCES
1.
Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: Results of linkage with the California cancer registry. Am J Obstet Gynecol 2003;189(4):1128–35. [CrossRef]
[Pubmed]

2.
Bachanova V, Connors JM. Hodgkin lymphoma in pregnancy. Curr Hematol Malig Rep 2013;8(3):211–7. [CrossRef]
[Pubmed]

3.
Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin 2018;68(2):116–32. [CrossRef]
[Pubmed]

4.
Küppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J Clin Invest 2012;122(10):3439–47. [CrossRef]
[Pubmed]

5.
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127(20):2375–90. [CrossRef]
[Pubmed]

6.
7.
Allin D, David S, Jacob A, Mir N, Giles A, Gibbins N. Use of core biopsy in diagnosing cervical lymphadenopathy: A viable alternative to surgical excisional biopsy of lymph nodes? Ann R Coll Surg Engl 2017;99(3):242–4. [CrossRef]
[Pubmed]

8.
Zanotti-Fregonara P, Jan S, Taieb D, et al. Absorbed 18F-FDG dose to the fetus during early pregnancy. J Nucl Med 2010;51(5):803–5. [CrossRef]
[Pubmed]

9.
Skoetz N, Will A, Monsef I, Brillant C, Engert A, von Tresckow B. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev 2017;5(5):CD007941.
[Pubmed]

10.
Walton JR, Prasad MR. Obstetric and neonatal outcomes of cancer treated during pregnancy. Clin Obstet Gynecol 2011;54(4):567–73. [CrossRef]
[Pubmed]

11.
Ebert U, Löffler H, Kirch W. Cytotoxic therapy and pregnancy. Pharmacol Ther 1997;74(2):207–20. [CrossRef]
[Pubmed]

12.
Doll DC, Ringenberg QS, Yarbro JW. Antineoplastic agents and pregnancy. Semin Oncol 1989;16(5):337–46.
[Pubmed]

13.
Vandenbroucke T, Van Calsteren K, Amant F. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med 2016;374(7):693. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
I would like to acknowledge Dr. Norra Harun, pathologist in Hospital Tengku Ampuan Afzan, Kuantan and Dr. Kim Kwan Tan, pathologist from Hospital Pakar Sultanah Fatimah, Muar for their contributions in providing the images for the above patients.
Author ContributionsSopian Abdul Wahab - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Roziah Husin - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hafliza Salleh - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ahlam Naila Kori - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Sopian Abdul Wahab et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.