|
Review Article
Validation of Sinhala and Tamil translations of the motherisk: Pregnancy unique quantification of emesis (PUQE) scoring system
1 Postgraduate Institute of Medicine, University of Colombo, Colombo, Sri Lanka
Address correspondence to:
Thilina Maduranga Liyanage
Postgraduate Institute of Medicine, University of Colombo, Colombo,
Sri Lanka
Message to Corresponding Author
Article ID: 100036G06TL2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Liyanage TM. Validation of Sinhala and Tamil translations of the motherisk: Pregnancy unique quantification of emesis (PUQE) scoring system. Edorium J Gynecol Obstet 2024;9(1):9–23.ABSTRACT
Aims: Nausea and vomiting in pregnancy (NVP), where there are no other underlying causes, is one of the most common symptoms in the early part of the pregnancy. It is responsible for up to 80% of pregnant population. By using PUQE score system, it is very convenient to categorize pregnant women with nausea and vomiting come to outpatient department (OPD) or general practice into those who can manage in community setting or those who need inpatient treatments. Objectives: Validation of PUQE instrument to assess NVP and hyperemesis gravidarum (HG) women in Sri Lanka.
Methods: Descriptive cross-sectional study conducted among 160 pregnant women presented to the Gynecology Unit of Teaching Hospital Anuradhapura (ATH) during three months duration. Translated self-administered PUQE questionnaire was applied to the patients with signs and symptoms with emesis in pregnancy. All study participants were undergoing clinical judgment as a confirmation test. Diagnostic test accuracy methods were used to assess criterion validity of the experimental tool. Data were analyzed by using SPSS statistical software version 25.0.
Results: Study population has a mean age of 27.96 (SD 5.31). Majority of study participants were Sinhalese and according to the parity majority were primigravida pregnancies. Majority of study participants were diagnosed as NVP Stage II (N=134:53.8%) by the PUQE scoring system. According to the clinical judgment Stage II of NVP was diagnosed among 127 (79.4%) study participants. Mean score of the PUQE scale was 9.08 (SD=2.35). Reliability assessment of the PUQE scoring system was reported as an excellent level (Cronbach alpha=0.804). Five types of management strategies were detected among study participants. Significantly high percentage of study participants were given first line antiemetics and oral rehydration solutions. Calculated sensitivity of the PUQE scoring system to diagnose Stage I of NVP was 69.56% and specificity was 96.35%. Calculated sensitivity of the PUQE scoring system to diagnose Stage II of NVP was 100% and specificity was 78.78%. All pregnant women who were clinically detected as Stage II NVP patients were detected as Stage II NVP patients by the PUQE scoring system also. None of the false positives and false negatives regarding Stage II of NVP detected by the PUQE scoring system.
Conclusion: First line antiemetics and oral rehydration solutions are usually used for treating pregnancy-related nausea and vomiting. Stage II NVP conditions were detected with a higher prevalence when NVP status was diagnosed clinically and with PUQE scoring system. Specificity of PUQE scoring system appeared higher while detecting NVP Stage I and for NVP Stage II, sensitivity of PUQE scoring system appeared high. For Stage III NVP, clinical judgments and PUQE scoring system values were completely compatible. Management strategies used for treating pregnancy-related hyperemesis status should be further studied. Conducting these studies at different study settings may help to achieve more successful results. Pregnancy unique quantification of emesis tool can be used to diagnose NVP status more descriptively. Also, this PUQE tool is a valid tool which can be effectively used for patient management. Application of PUQE scoring system should be published by planning and implementing staff awareness programs for both curative sector and public health sector.
Keywords: Emesis, PUQE scoring system, Validation
INTRODUCTION
Nausea and vomiting in pregnancy (NVP) is one of the most common symptoms in the early part of the pregnancy. It presents in up to 80% [1] of the pregnant population. Simple nausea is prevalent in about 50–80% of pregnancies, and vomiting and retching in about 50% of them [1]. Retching, in the absence of expulsion of the gastric contents is a distinct symptom. “The misnomer, morning sickness,” collectively describes retching with NVP, contradicts the point that “symptoms can occur at any part of the day.”
These symptoms are usually started early in the period of gestation (POG), which is the first trimester at around 4–7 weeks of gestation period. If it starts after 10 weeks and 6 days of POG, other possible causes such as gastroenteritis, gastric ulcers, cholecystitis, appendicitis, pyelonephritis, neurological, and metabolic abnormalities should be excluded [2],[3],[4],[5]. Incidence is peak at 9 weeks of gestation and by the time of 20 weeks, over 90% of women’s symptoms would have been settled. In 10–20% of women symptoms persist beyond this time [6].
Hyperemesis gravidarum (HG) is the severe form but it is less common (0.3–3.6% of pregnancies). It is responsible for significant morbidity and contributes to repeated incidence of hospital admissions with a typical stay around 3–4 days [7],[8],[9]. Recurrence of HG rates may differ “from 15.2% in a Norwegian hospital registry study 8 to 81% using self-reported diagnosis.” The occurrence of NVP and HG diminishes in subsequent pregnancies if paternal factor is changed (10.9%) when compared to no change [16%; adjusted odds ratio (OR) 0.6, 95% confidence interval (CI) 0.39–0.92) [10],[11].
Pathophysiology of emesis in pregnancy
There are several theories for what may contribute to the development of HG and NVP. These may range from the feto-protective and immunological to genetic, biochemical, and bio-social.
Levels of human chorionic gonadotropin (hCG) have been implicated. hCG levels peak during the first trimester, corresponding to the typical onset of hyperemesis symptoms. It is correlated with maternal disorders with raised hCG levels, such as multiple pregnancy and trophoblastic disease [6],[12]. However, these data have not been consistent [13].
It is also assumed that estrogen also contribute to nausea and vomiting in pregnancy. Estradiol levels increase early in pregnancy and decrease later, mirroring the typical course of NVP. Additionally, it is a known side effect of estrogen-containing medications. As the level of estrogen increases, so does the incidence of vomiting [6].
It is well-known that the lower esophageal sphincter relaxes during pregnancy due to the elevations in estrogen and progesterone. This leads to an increased incidence of gastroesophageal reflux disease (GERD) symptoms in pregnancy, and one symptom of GERD is nausea [2]. Studies examining the relationship between GERD and emesis in pregnancy report conflicting results. An increased risk of hyperemesis gravidarum has been demonstrated among women with family members who also experienced hyperemesis gravidarum.
Two genes, GDF15 and IGFBP7, have been potentially linked to the development of hyperemesis gravidarum [14]. Several robust evidences support a genetic contribution to NVP. Only one study conducted on NVP in twins and according to this, the concordance rates were more than twice as high for monozygotic compared to dizygotic twins [15]. Various studies revealed that mothers and siblings of patients are more likely to be affected than those of unaffected controls [16],[17]. Nausea and vomiting in pregnancy is common in patients with genetically related diseases such as glycoprotein hormone receptor errors, defects in gestation, or errors in fatty acid transfer or mitochondrial oxidation [18]. Overall, these data highlight the genetic contribution to NVP.
Several observational studies have reported conflicting information regarding the incidence of low birth weight and premature infants in the setting of NVP [19],[20]. However, studies have not shown an association between hyperemesis and perinatal or neonatal morality [19],[20],[21]. The frequency of congenital anomalies does not appear to increase in patients with hyperemesis [20].
Complications associated with hyperemesis in pregnancy
The health status of the pregnant women with their family, social and occupational functions, is affected by the severity of NVP [19]. The negative effect on fetal growth may manifest as low birth weight [20].
Severe or prolong NVP leads to HG with inadequate nutrition or dehydration. This would result ptyalism (inability to swallow saliva), marked loss of weight (LOW), muscle wasting, ketonuria, dehydration, and electrolytes imbalance including hypokalemia and metabolic hypochloreimic alkalosis [22],[23]. However, hospitalization is not included in the definition of HG, as occasionally, it may be controlled by outpatient interventions [22],[23].
The operational definition of HG considers the effects of the vomiting such as LOW, dehydration and ketosis. Pathological causes must be excluded prior to the diagnosis of NVP. Hyperemesis gravidarum leads to fetal growth restriction (FGR), central pontine myelinolysis due to hyponatremia in mother and Wernicke’s encephalopathy (WE) due to thiamin deficiency [24],[25],[26] and higher rates of depression and anxiety during pregnancy [24],[25],[26],[27],[28]. Due to repeated bouts of forceful nausea and vomiting, there have been case reports of injuries such as esophageal rupture and pneumothorax [29].
Severity of the HG depends on the frequency of vomiting, duration of symptoms, and the presence of the evidence of encephalopathy like headache, confusion, and altered level of consciousness [24],[25]. Signs of severity include Jaundice, LOW more than 10% of previous body weight, altered thyroid functions with elevated free thyroxine (fT4) and low thyroid-stimulating hormone (TSH), elevated liver transaminases, signs of dehydration, and evidence of WE. The classic triad of symptoms in WE are ophthalmoplegia (later expanded to other eye movement disorders) [24],[25],[26] ataxia (later expanded to any cerebellar signs) [24],[25] and confusion (later expanded to other mental changes) [27].
Justification
The myths or the disbeliefs in the general community on NVP have their own implications on the outcome. It is believed that morning sickness occurs only in the morning, only in the first trimester, only in first pregnancy and it signals the presence of a healthy baby. Skipping meals due to the fear of vomiting would worsen the outcome.
In Sri Lanka, it is very important to have a questionnaire to diagnose and quantify patients with NVP and hyperemesis gravidarum because most of the pregnant mothers who get NVP tend to seek treatment like local remedies, ayurvedic treatments, or by doing rituals which ultimately results in adverse fetal and maternal complications. Despite the magnitude of the problem in Sri Lanka, the number of studies is highly insufficient to express the actual burden.
By using PUQE score system, it is very convenient to categorize patients with nausea and vomiting come to OPD or general practice into those who can manage in community setting or those who need inpatient treatments. Inward patients can be categorized and managed according to the severity (mild, moderate, and severe). It is also useful to assess the response and further follow-up at the ward as well as following discharge.
This study assists the health professionals, researchers, affected patients and government authorities to unfold the problem. Additionally, it dispenses background statistics for future studies.
LITERATURE REVIEW
Assessment of hyperemesis in pregnancy
Validated methods for the assessment of severity of NVP include the PUQE index and the Rhodes index. Initially the chemotherapy patients were assessed by the Rhodes index in 1984 [30],[31],[32], which includes evaluation of clinical symptoms and the subsequent stress [30],[31]. It has highlighted the psychosocial morbidity of nausea and vomiting in patients obtaining chemotherapy for cancer [30],[31],[32]. Later it was validated for NVP [30],[31]. Since the establishment of first validation tool, “Motherisk-NVP Health line” group utilized the Rhodes’ scoring system on many mothers with NVP and have found that system to be time-consuming and tedious [30],[31].
Assessment of severity of NVP involves the assessment of the stress caused by each symptom. As this was well preserved in the measurements taken by Rhodes score, it was suggested that stress measures can be eliminated without affecting the qualitative aspect of the score.
The assessment of NVP using Rhodes’ score has several shortcomings: It measures the length and the frequency of nausea during last 12 hours. “Least square regression analysis has revealed a very significant correlation between the length of nausea per 12 hours and the number of bouts of nausea (r = 0.86; p < 0.0001)” [30],[31],[32]. In addition, the change in severity of nausea over the time is accurately correlated with altering the frequency of nausea (r = 0.95; p < 0.0001) [30],[31],[32]. Therefore, it was unjustifiable to add a score of the same feature twice.
This research was designed to develop a clinically applicable, simplified score for the quantitative analysis of the severity of NVP. The sensitivity to variation of the symptoms with time and the score itself can be compared with the “validated Rhodes’ score.”
Approximately 59,000 pregnant women are hospitalized with NVP and HG annually in the United States, with an incidence of 0.5% [33],[34],[35],[36]. Incidence of NVP may vary from 0.3% in a Swedish study to a higher value such as 10.8% in a Chinese study of pregnant women [30],[32]. Ethnic variations may influence the incidence of HG. A study conducted in Norway from 1967 to 2005 revealed the prevalence of HG as 0.9%, but when broken down by ethnicity [37] it was 1.9% in Turkish women and 2.2% among Pakistani women [36].
A study of “birth and death certificates in California after 20 weeks of POG associated with neonatal hospital discharge details” in 1999 with documented HG revealed an incidence of 0.5% (2,466 cases out of 520,739 births) [38]. These mothers were notably non-Hispanic or non-white [38]. A study in Canada revealed HG among 1270 mothers (0.8%) from 156,091 women having singleton pregnancies from 1988 to 2002 [38]. Above data were reassessed and clarified in another study done in Canada related to the same period of the Atlee perinatal database of Nova Scotia, based on the gestational population over 20 weeks, which revealed 1,301 cases (0.8%) among 157,922 pregnancies [38]. Asian mothers show higher prevalence of HG. For instance, a study carried out in Malaysia detected 192 cases (3.9%) out of 4,937 pregnancies [38]. Furthermore, a study of 3,350 singleton childbirths in an East Asian population revealed HG in 119 (3.6%) of the population [38]. An evaluation of 1,867 singleton pregnancies in Shanghai, China, in the period of 1986–1987, showed the highest incidence of NVP (10.8%) [38]. Unlike others this study utilized prenatal medical records of significant vomiting, rather than hospital admission for HG. Therefore, it included patients with chronic liver failure, chronic arterial hypertension, chronic renal failure, and pre-eclampsia.
Native Indian and Sri Lankans have three times higher risk of experiencing severe NVP or HG than native Norwegians. This result derives from the study by the “Norwegian Institute of Public Health” on 900,000 primp pregnancies registered for the first time in Norway’s birth registry over period of 40 years [37].
Previous studies have shown that 90% of pregnant women have nausea and vomiting to some extent, while 0.5–2% has HG. This could be fatal for the mother as well as child due to lack of adequate fluid, electrolytes, and nutrition, if untreated. In the United States, it is the most common cause of hospitalization in early pregnancy. The cause of HG is unknown.
Norwegian obstetrician in the epidemiology division wanted to evaluate if the country of birth of mothers influenced the prevalence of HG. In an article “Variations in the prevalence of HG by country of birth: a study of 900,074 pregnancies in Norway, 1967–2005” [37], Vikanes gathered details from the “Norwegian Birth Registry,” which records all pregnancies and complications of pregnancy since 1967 [37]. There were 300 cases of HG on 900,000 pregnancies, with a general prevalence of 0.89%. The details on birth country and educational qualifications of the mother were recorded by “Statistics Norway” and are linked to pregnancy details on from the “mother’s unique personal identification number [37].” Socio-demographic data such as birth country, age, marital status, schooling, and number of fetuses at each pregnancy have also been evaluated. Vikanes says “This is one of the largest studies on HG. In contrast to earlier studies we tested the quality of the data and therefore have confidence in our findings” [37].
Native Indian and Sri Lankan mothers had the highest prevalence of HG (3.2%), followed by Africans (3.1%) “Excluding North Africa” and mothers from Pakistan (2.1%). The Norwegians (0.9%), Western Europeans (0.8%), and North Americans (0.9%) had minimum prevalence rates [37],[38]. Women aged between 20 and 24, married, with one fetus or more were the high-risk socio-demographic features related to greater risk of HG [37],[38].
Vikanes says “The difference in prevalence of HG related to the mother’s country of birth cannot be explained by differences in socio-demographic characteristics. We have to look for other explanations such as genetic factors, a change of diet or a history of infections. This topic needs further research to identify ways to prevent this life-threatening and distressing condition” [37],[38].
Although the accurate etiology behind NVP is not known, it is widely accepted that this is a multi-factorial entity with physiological, genetical, nutritional, and psychosocial determinants [39],[40]. However, the claims of these determinants remain controversial and are often limited to the first trimester or HG [39],[40]. Clinical features may vary greatly and are influenced by “age, marital status, area, ethnicity, cultural, social, and educational status of the individual” [39],[40]. Studies have revealed that anxiety, stress, depression, unwanted pregnancies, consumption of cigarettes, alcohol, and drugs, can have negative effects on the well-being of the fetus, child, and mother of hyperemesis [41].
Data from the “Hyperemesis Education and Research Foundation” have shown that NVP and HG can value at least $200 million a year for hospital admissions in the United States [42],[43]. In a similar economic analysis, Piwko et al. predicted that the United States will spend nearly $2 billion on NVP [42],[43]; 60% of these expenses are attributable to direct costs (drugs and human resources) and 40% to indirect costs (time lost at work) [42],[43].
According to the data originated from German, the annual cost of hospitalization for HG was nearly 28 million Euros. The value of human hours lost at work and outpatient care are not considered in this figure [42],[43]. It is increasingly evident that NVP has become a common occurrence among the urbanized pregnant women, characterized by greater genetic heterogeneity, as its presence is rare in the foraging population, such as “the Bushmen of South Western Africa and the Amazonian Amerindian tribe with good genetic homogeneity” [36],[37],[38],[44].
In addition to the ethnic variability, some demographic and anthropometric factors were also related to NVP, since the studies showed that a relationship with parity, gender of fetus, maternal weight and age, family history, and past history of NVP that supports the risk of severe NVP [36],[37],[44].
Nausea and vomiting in pregnancy and HG may be related to severe intrauterine growth restriction or death [45],[46],[47]. Intense and prolonged vomiting can cause “tears in esophageal mucosa (Mallory–Weiss syndrome), rupture of the spleen or esophagus, choroid bleeding, transient hypothyroidism, pneumothorax and Wernicke encephalopathy causing neurological complications due to lack of vitamin B1” [23],[24],[25],[26],[28],[29].
Null parity, prim gravidity, food aversion, and extreme salivation were notably related to NVP [38],[39]. Other significant factors related to severity and hospitalization are “being unmarried, loss to work, affected relationship, more frequent vomiting, and early onset of symptoms” [38],[39].
Evidence for management practices
Based on a Cochrane library review [48], various systematic reviews and meta-analyses [49],[50],[51] and observational studies data[51] have informed about effectiveness and safety of a lot of antiemetic used for NVP and HG, and found out no higher risk of fetal anomalies or any kind of adverse pregnancy outcomes. Above-mentioned drugs are antihistamines (cyclizine, cinnarizine, doxylamine [52], promethazine, and dimenhydrinate); phenothiazine (prochlorperazine, perphenazine, and chlorpromazine); and dopamine receptor antagonists (metoclopramide and domperidone) [53].
Various classes of antiemetics can have various mechanisms of action and as a result of that synergistic effect; drug combinations can be useful in poor response to a single drug. Refractory vomiting causes poor absorption of drugs from the alimentary system. Intravenous, intramuscular, subcutaneous, or rectal routes may be more effective in these patients.
According to American College of Obstetrics and Gynaecology (ACOG) guidelines for nausea and vomiting in pregnancy, initial treatment should begin with non-pharmacologic interventions such as switching the patient’s prenatal vitamins to folic acid supplementation only, using ginger supplementation (250 mg orally 4 times daily) as needed [54],[55],[56], and by applying acupressure wristbands [57],[58],[59]. If the patient continues to experience significant symptoms need hospital admission. Some recent evidence favors of vitamin B6 (pyridoxine) and doxylamine (pyridoxine only) has no evidence [48],[60],[61]. Therefore Royal Collage of Obstetrician and Gynecologist (RCOG) has not recommended. Three dosing regiments are endorsed by ACOG, including pyridoxine 10–25 mg orally with 12.5 mg of doxylamine 3 or 4 times per day, 10 mg of pyridoxine and 10 mg of doxylamine up to 4 times per day, or 20 mg of pyridoxine and 20 mg of doxylamine up to 2 times per day. As demonstrated in multi-center randomized controlled trials, these first-line medications demonstrate efficacy in the treatment of nausea and vomiting, preserved good fetal and maternal safety profiles and are listed as one of the few FDA Pregnancy category A drugs [62].
Second-line medications (first-line antiemetics) include antihistamines and dopamine antagonists such as oral dimenhydrinate 25–50 mg 4–6 hourly, oral diphenhydramine 25–50 mg 4–6 hourly, rectal prochlorperazine 25 mg 12 hourly, or rectal/oral promethazine 12.5–25 mg 4–6 hourly [48],[49],[50],[51],[52]. If the patient continues to experience significant symptoms without exhibiting signs of dehydration, metoclopramide, ondansetron, or promethazine may be given orally [63],[64],[65],[66],[67],[68],[69],[70]. In the case of dehydration, intravenous fluid boluses or continuous infusions of normal saline should be given in addition to intravenous metoclopramide, ondansetron, or promethazine [63],[64],[65],[66],[67]. Electrolytes should be replaced as needed. Severe refractory cases of HG may respond to intravenous or intramuscular chlorpromazine 25–50 mg or oral/intravenous methylprednisolone 16 mg 8 hourly [71],[72],[73],[74],[75].
Metoclopramide should be used as second-line therapy due to the risk of short-term extrapyramidal effects and tardive dyskinesia [63]. The evidence for these side effects especially in younger population was reinforced by “A review of metoclopramide, conducted by the European Medicines Agency’s Committee for Medicinal Products for Human Use” [63],[64]. Its recommendations include use of short-term metoclopramide (maximum dose; 30 mg within 24 hours or 0.5 mg/kg body weight within 24 hours for maximum 5 days) [64] and slow intravenous injection over 3 minutes as it has less risk of dystonic reactions [64].
Ondansetron has a mixed safety profile. A retrospective evaluation of data from “Danish birth registry of 607385 pregnant women” revealed less risk of major congenital defects, preterm births, stillbirths, and small for gestational age (SGA) [65]. Another case control study involving 4,521 cases and 5,858 controls revealed threefold higher risk of cleft palate (adjusted OR 2.37, 95% CI 1.18–4.76) [66], but authors suggested that this might be due to a chance as large amount of cases evaluated. Analysis of data derived from “Sweden Medical and Birth Register” [67] revealed elevated risk of atrial septal defects (ASD) and ventricular septal defects (VSD) (OR 1.62, 95% CI 1.04–2.14, and risk ratio 2.05, 95% CI 1.19–3.28, respectively). Hence ondansetron could be recommended to patients who responds poorly to first line antiemetics and preferably after first trimester. According to four randomized controlled trials (RCTs) comparing ondansetron with doxylamine, maxalone, and pyridoxine [68],[69],[70], ondansetron was more effective and possesses less side effect profile [70].
Current evidence does not reveal relationship between NVP and vitamin B6 levels in first trimester [60]. Another Cochrane review found that use of pyridoxine as a therapy for NVP bears insufficient evidence [48]. A “RCT on use of pyridoxine in HG” [47] did not reveal reduction in hospital admissions or improvement in symptoms when they were given pyridoxine with intravenous crystalloids intravenous metoclopramide and oral thiamine. The control group was given a placebo in addition to standard therapy. A similar non-RCT [62] concluded that both doxylamine and pyridoxine were significantly more effective than pyridoxine alone.
Corticosteroids has a major role in therapy for NVP and gives rise to a dramatic improvement in “case series of women with severe HG refractory to conventional therapies” [71]. Different RCTs reveal conflicting evidence [72] and the largest RCT could not show reduction in re-hospitalizations [73],[74]. The reasons behind these differences may include various routes of drug administration and varying degree of severity of the cases where severe cases show better response to corticosteroids. According to another double-blind RCT [75], 300 mg of intravenous hydrocortisone daily dose was more effective than intravenous metoclopramide to reduce the severity and the recurrence in intensive care unit (ICU) patients with NVP and HG.
Corticosteroids are recommended in patients who failed to respond to conventional therapy. It can be commenced with 100 mg 12 hourly, which can be gradually converted to oral daily dose of prednisolone (40–50 mg). The lowest possible dose that controls the symptoms must be used as the maintenance until the period where symptoms are resolved [76].
Randomized controlled trial (RCT) examined 50 patients with HG, where they are managed with vitamins, 5% Dextrose, 0.9% saline, and randomly allocated diazepam [77]. Patients who are treated with diazepam had less nausea, but no difference with the occurrence of vomiting. But still diazepam is not recommended.
Nausea and vomiting in pregnancy and HG-related metabolic abnormalities include hypochloremia, hyponatremia, hypokalemia, and ketosis. Therefore, volume replacement with intravenous fluids and replacement of electrolytes plays a pivotal role in the management of NVP and HG. But the choice of fluid is still questionable. National Institute for Health and Care Excellence (NICE) clinical guideline 174 provides outline of fluid management [78] Wernicke’s encephalopathy will be precipitated by the administration of Dextrose containing fluids especially with thiamine deficiency. Therefore, high dose (100 mg) parenteral thiamine should be given prior to dextrose administration.
OBJECTIVES
General objective
Validation of PUQE instrument to assess NVP and HG women in Sri Lanka.
Specific objectives
To validate PUQE instrument for the assessment of NVP and HG among pregnant women attending the hospitals in Sri Lanka.
- To describe management practices for NVP and HG among mothers admitted to Teaching Hospital Anuradhapura (ATH).
- To assess the tool among patients admitted with NVP and HG.
- To categorize patients admitted to hospital due to NVP.
MATERIALS AND METHODS
Study design
This study is cross-sectional descriptive study. The study is planned as a hospital-based study which is done in ATH.
Study setting
The study was conducted in ATH. This is the largest hospital in North Central province of Sri Lanka, situated in Anuradhapura town in the district of Anuradhapura.
District of Anuradhapura has one teaching hospital, three base hospitals, 40 divisional hospitals, 12 primary health care units, 20 medical officer of health (MOH) offices, and 5 other health institutions, providing their service to a population of 856,232 (consensus 2012) apart from considerable amount of patient drainage from surrounding districts like Matale, Puttalam, Vavuniya, and Kurunegala.
Teaching Hospital Anuradhapura and Base Hospital Thambuththegama have Obstetrics and Gynecology units under care lead by consultant Obstetrician & Gynecologists whereas Padaviya Hospital is currently covered by a consultant from ATH.
Total number of wards in ATH is 68. It consists of 3 Obstetrics and Gynecology units (1 professorial unit—No. 68 and 2 health ministry units—No. 23A and 23B) and strength of 68 beds in 3 gynecology wards (Table 1).
Every year considerable number of patients admits with nausea and vomiting in pregnancy and hyperemesis gravidarum.
Study population
This contains pregnant women in their early part of pregnancy period with NVP and HG (first trimester and second trimester up to 16 weeks of POG) to abovementioned gynecological wards in ATH.
Study period
It was expanded from the date of starting the study until the time of writing this article.
It was nearly two and half years period starting from September 2016. Data collection was planned to carry out for period of three months starting from November 2016 to January 2017, but it was delayed due to various unavoidable reasons.
Inclusion criteria
- Pregnant women admitted with nausea and vomiting in pregnancy and hyperemesis gravidarum to all gynecological wards in ATH.
- Patients who were interviewed by the investigators and consulted the relevant specialist.
Exclusion criteria
- These include patients who were too ill to be interviewed.
- Any other reasons like mentally subnormal patients and patients who were deaf and dumb.
- Any pregnant woman of gestational age was more than 16 weeks.
- Any pregnant woman who could not understand Sinhala or Tamil languages.
- Pregnant women who experienced nausea and vomiting due to other diseases like acute gastroenteritis, liver disease, gall bladder disease, acute appendicitis, etc.
- Patients who were transferred to other hospital for further medical management related to index problem or any other problem.
- Women who left the wards against medical advice.
- Those who went missing just after admission.
- Where the questionnaire was filled but it was not possible to complete the assessment by the consultant.
Sampling and sample size calculation
Using PUQE scoring system, it is possible to identify three groups of patients based on the severity of nausea and vomiting in pregnancy in the study population (i.e., patients with mild, moderate, and severe categories).
The required minimum number of pregnant women with nausea and vomiting (n1) is calculated as
n1 = 4 Z2a P (1−P)/W2
Z is the standard normal deviation for the selected level of confidence > 1.96 for a confidence level of 95%, P is the expected sensitivity of PUQE score (can take this 90% since there are no previous studies available on the sensitivity of the questionnaire), and W is the required level of precision (can take this as 0.10 or as 0.15).
Thus, calculation will be provided that the prevalence of NVP among all pregnant mothers around 80%.
n1 = 4×1.96 × 0.90 (1–0.90)/0.15 2 = 61.47 (Nearly 62)
Then the number of pregnant mothers included in to the study
N = 62 × 100/80
N = 78 (assuming that there could be an expected nonresponse rate of 10%, need to add 10% to the number you get for the above calculation).
Selection of the study sample
All pregnant women admitted with nausea and vomiting were recruited to the study, subjected to the exclusion criteria. Total numbers of 160 patients were identified during the study period of three months.
Study instruments
Perpetration of the questionnaire
Data were collected using an interviewer-based questionnaire. This questionnaire consisted of two parts. These parts included questions pertaining to socio-demographic factors and appropriate Sinhala and Tamil translations of the PUQE score.
The English version of the scale was available for use whenever necessary. Questionnaire included close-ended questions which were simple and easy to understand anyone.
The information required included basic socio-demographic data, details pertaining to nausea and vomiting in pregnancy.
PUQE scoring system
This scoring system is an interviewer administered scale, which consists of three main components. Each component has five options related to nausea and vomiting severity in last 24-hour period. Three main areas considered in the score are about nausea, vomiting, and retching or dry heaving. The minimum value which could be obtained using the score is 3 and the maximum score is 15. Categorization of nausea and vomiting in pregnancy based on the total score of the PUQE score less than 6 is considered as mild hyperemesis. Total score between 7 and 12 is considered as moderate hyperemesis and total score between 13 and 15 is considered as severe hyperemesis.
Translation of the PUQE
The back-translation technique was used to achieve semantic equivalence (Flaherty 1998). First, a person fluent in both English and Sinhala and another person fluent in English and Tamil translated PUQE score from English to Sinhala and Tamil, respectively. Second, the instrument was back translated from respective languages to English language by another independent language expert. Third, two versions were compared to identify same meaning by another separate language expert. As there were some minor differences, some wards were altered (rewarding was done) to obtained the maximum effect. The process was repeated until the same meaning was obtained in the translated version. For this process it took some time to complete. Language experts were mean, government authorized language translators.
Assessment of Judgmental Validity-For content and consensual validity of the translated version of PUQE score was assessed by a multidisciplinary panel of experts comprising of a Consultant Obstetrician, Consultant Physician, and Community Medicine Expert in teaching hospital Kandy to minimize the bias. Each questionnaire was evaluated by using a rating system.
Pre-testing of the PUQE score
Sinhala and Tamil translated versions of PUQE score were administered to 10 from each language speaking women who were not included in to the study proper. Pre-testing was done to identify whether the patients could easily understand the questions that were put on them, the acceptability, and the time taken to administer the questionnaire and difficulties with administering it.
Pilot study
The questionnaire was administered to 20 patients of each language separately by the principal investigator and Tamil speaking medical officer. For pilot study, Teaching Hospital Kandy was selected. All necessary permission was taken from relevant authorities. Inclusion and exclusion criteria were the same in the proper study. Aim was to assess the feasibility of the study and to determine the acceptability of the score. Possible inappropriateness due to cultural differences was excluded. All items in the PUQE score were able to retain. Minute alterations had to be made and those were adjusted with the approval of the supervisor. It is also enabled the author to identify possible problems related to data collection.
Administering the PUQE score
Translated (Sinhala and Tamil) PUQE score was administered by the principal investigator to Sinhala speaking women and Tamil speaking medical officer (Relief House Officer) to Tamil speaking women.
The approval from director and the consultants of the relevant wards was taken to carry out the study in Teaching Hospital Anuradhapura. The cooperation of the medical and nursing staff was obtained by explaining the objectives of the study.
Assessment was done when the patient was clinically capable to interview. This interview was usually carried out usually within 24 hours after admission and in some instances, it extends to 48 hours due to various unavoidable reasons which was not significantly affected the accuracy of the study.
Interview took place in the relevant gynecology wards. Privacy was ensured, so the interview could be carried out in an undisturbed manner. Every effort was made to ensure that the environment was appropriate for such an interview. Each Sinhala speaking patients were interviewed individually by the principal investigator and Tamil speaking patients were interviewed by relevant medical officer. Introduction about the principal investigator and the objectives of the study were explained. It was very important to develop good rapport and investigators were able to do so. Confidentiality was assured. Consent was obtained after explaining the purpose of the study. Patients were informed that not giving consent to participate in the study would not affect their treatments. Consent forms were available in Sinhala, Tamil, and English. Those who could not write gave their consent verbally and by placing their thumb imprints. We did not encounter patients below 18 years. Average time taken to conduct the interview and administering the PUQE score was about 30 minutes. As a measure taken to minimize the errors of data collection, the PUQE score was administered by principal investigator.
Assessment of the severity of the nausea and vomiting by the consultant obstetrician
The second part of the assessment was the assessment of severity of nausea and vomiting by the consultant obstetrician. Both components of the assessment were completed on the same day, i.e., within 24 hours of admission. Principal investigator was able to carry out the interview most of the time before consultant’s severity assessment. Score of the PUQE was not revealed to the consultant.
Steps taken to ensure the quality of data
Confidentiality was stressed upon to improve the accuracy of the data that were collected. The purpose of the study was made clear. The assessment by the obstetrician was carried out as soon as possible (after clinically improved), within 24 hours of the assessment by investigators. The principal investigator and only one medical officer thorough in Tamil were the only data collectors to prevent inter-observer bias as much as possible. The PUQE score was pre-tested and the items in the PUQE score studied by panel of experts in the field to ensure acceptability. Validating the study instrument against the gold standard assured criterion validity.
Data analysis
A serial number was given to each patient at the end of the interview and the relevant data were entered in a Microsoft Excel worksheet by the principal investigator. The data were analyzed using SPSS software version 25.0.
The criterion validity of the PUQE score was tested as a screening instrument by calculating the sensitivity, specificity, positive and negative predictive values, and false positive and false negative rates for the NVP severity.
Positive predictive value
This is the probability that a person with a positive result actually has the disease. It indicates the probability that a patient is actually having significant NVP among patients with positive test results.
Negative predictive value
This is the probability that a person with a negative result does not have the disease. It indicates the probability that a patient is not having significant NVP among those with negative test results.
Likelihood ratio
This is the likelihood that a person with a disease would have a particular test result divided by the likelihood that a person without disease would have the result. An index of how good a test can be in contrast to the proportion of patients with and without significant NVP has given diagnostic test result.
Z test for proportion was used to compare categorical variables. Frequencies and percentages were used to describe the categorical variables. All continuous variables were described by using measures of central tendency. 0.05 probability cut-off and 95% confidence interval were taken for statistical significance.
Ethical considerations of the study
Informed written consent was obtained from every study participant. Those who do not read and write verbal consent and thumb imprint was obtained.
Confidentiality of the information obtained from the patient was ensured. Approval regarding collection of data was obtained from the directors of Teaching Hospital Anuradhapura and Teaching Hospital Kandy and consultants of the relevant wards. Approval has been taken from ethical review committee, Faculty of Medicine, University of Rajarata. Participants were informed that they had liberty to withdraw from the study at any time, if they wish to do so.
RESULTS
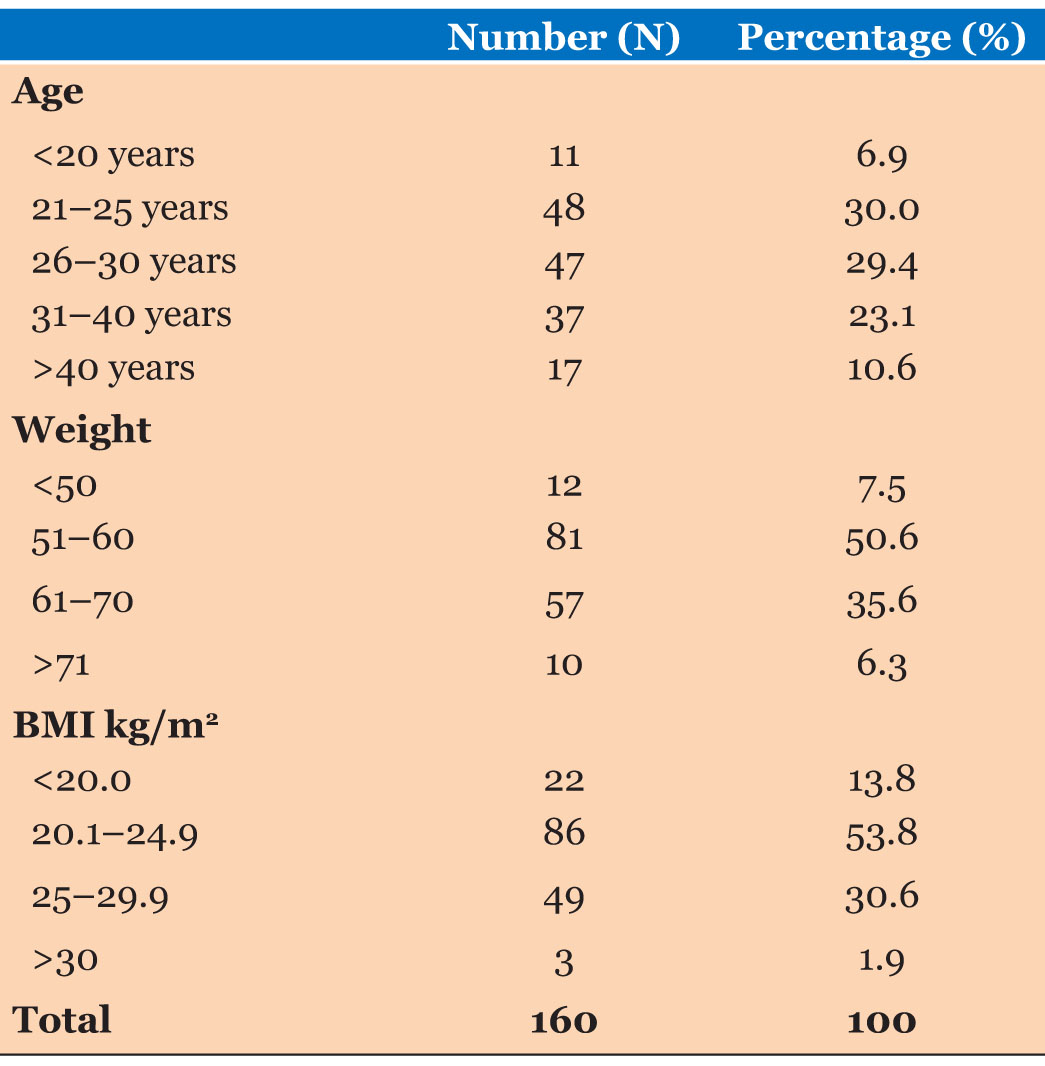
Ages of the study participants were ranged from 18 years to 39 years. Mean age of the participants was 27.96 years (SD=5.31). Highest number of the study participants represented 21–25 years age group. Body weight of the study participants were ranged from 47 to 79 kg. Mean body weight of the participants was 59.14 kg (SD=7.01 kg). Body mass index was ranged from 18.0 to 33.0 kg/m2. Mean body mass index was reported as 23.65 kg/m2 (SD=2.96 kg/m2) (Table 2).
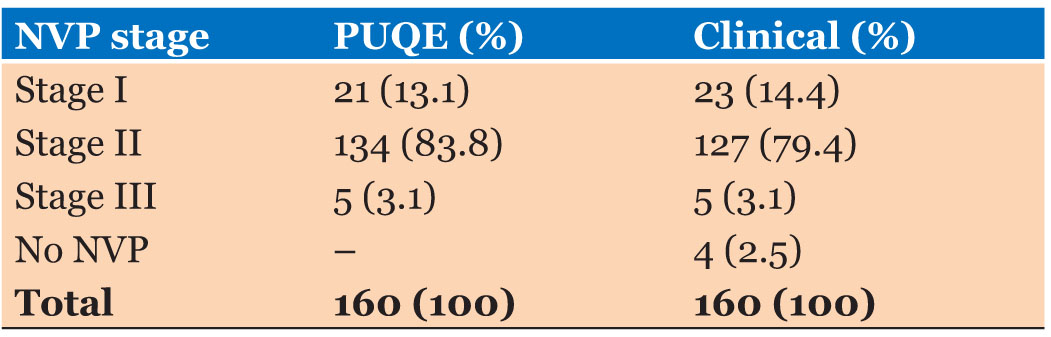
According to the results of the application of PUQE scoring system into the study participants, majority of study participants were diagnosed as NVP Stage II (N=134:53.8%). The clinical judgment Stage II of NVP was diagnosed among 127 (79.4%) study participants. Mean score of the PUQE scale was 9.08 (SD=2.35). Reliability assessment of the PUQE scoring system was reported as an excellent level (Cronbach alpha=0.804) (Table 3).
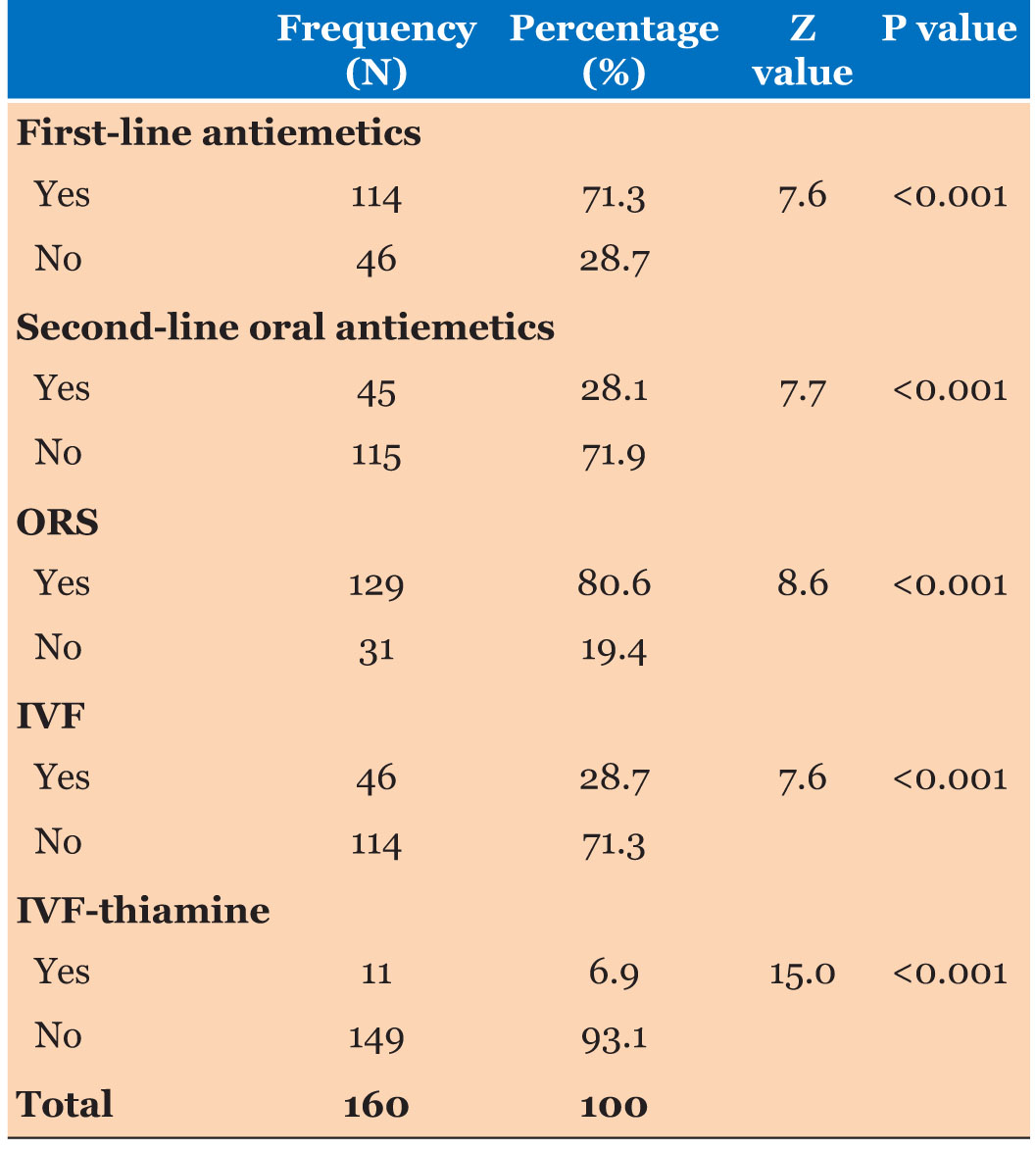
Five types of management strategies were detected among study participants. Significantly high percentage of study participants were given first line antiemetics and oral rehydration solutions. Significantly a smaller number of study participants were given second-line oral antiemetics. Intravenous fluids were given in a significantly low percentage of participants. Minimum number of study participants were given in vitro fertilization (IVF)-thiamine (Table 4).
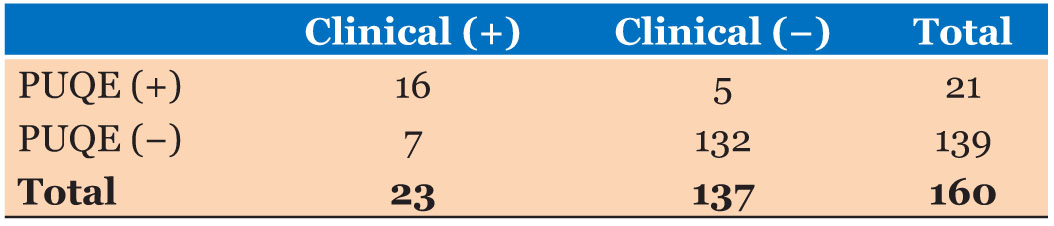
The criterion validity assessment compared to PUQE results and the clinical judgment are described in Table 5. Calculated sensitivity of the PUQE scoring system to diagnose Stage I of NVP was 69.56% and calculated specificity was 96.35%. The positive predictive value was calculated as 76.19% and the negative predictive value of the PUQE scoring system was calculated as 94.96%. The likelihood ratio of the PUQE scoring system as a positive test was 19 (95% CI=7.74–47.0). The likelihood ratio of the PUQE scoring system as a negative test was 0.32 (95% CI=0017–0.59).
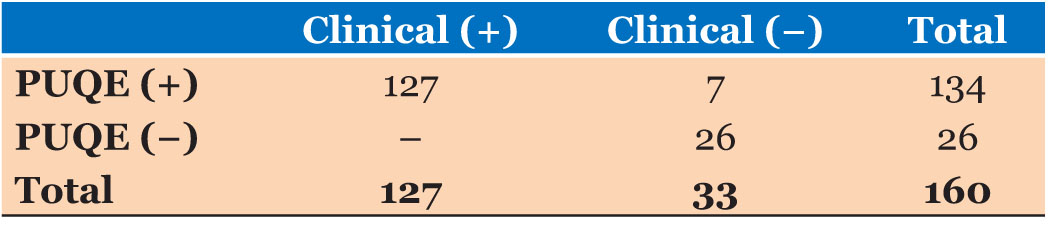
The criterion validity assessment compared to PUQE results and the clinical judgment are described in Table 6. Calculated sensitivity of the PUQE scoring system to diagnose Stage II of NVP was 100% and calculated specificity was 78.78%. The positive predictive value was calculated as 94.77% and the negative predictive value of the PUQE scoring system was calculated as 100%. The likelihood ratio of the PUQE scoring system as a positive test was 4.71 (95% CI=2.40–8.50). The likelihood ratio of the PUQE scoring system as a negative test was zero.
All patients who were clinically detected as Stage II NVP patients were detected as Stage II NVP patients by the PUQE scoring system also. None of the false positives and false negatives regarding Stage II of NVP detected by the PUQE scoring system.
DISCUSSION
Summary of results
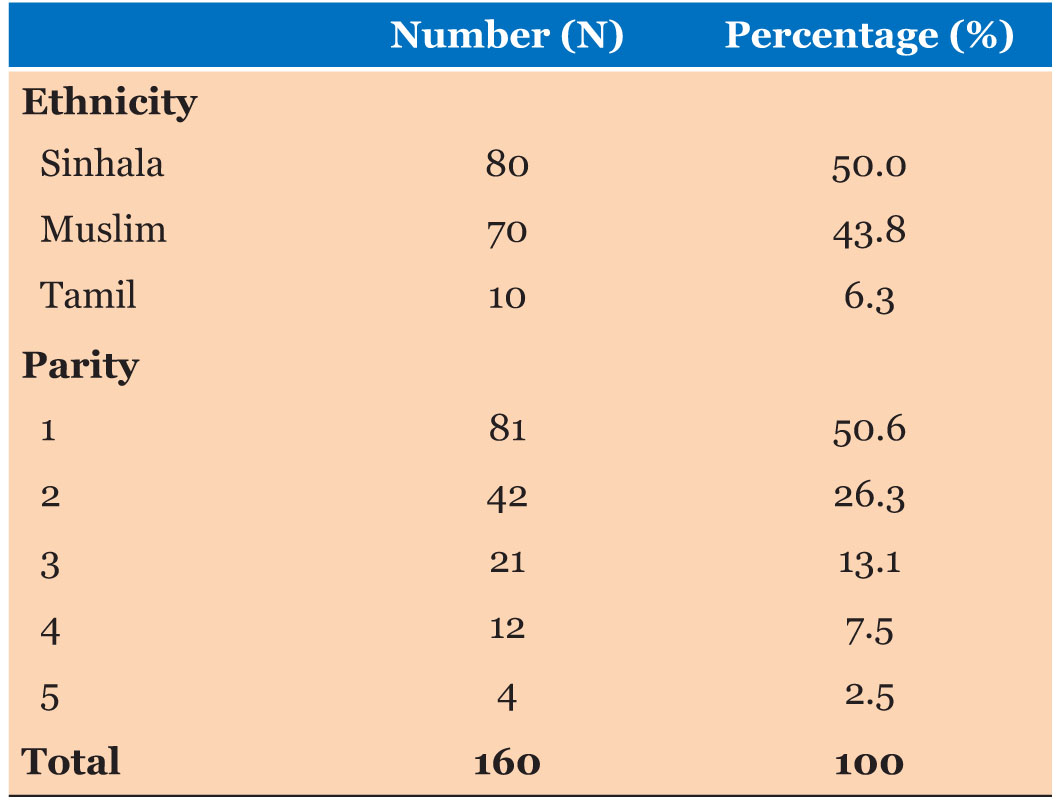
Ages of the study participants were ranged from 18 years to 39 years (Mean=27.96:SD=5.31). Body weight of the study participants were ranged from 47 to 79 kg (Mean=59.14:SD=7.01). Body mass index was ranged from 18.0 to 33.0 kg/m2 (Mean=23.65 kg/m2:SD=2.96 kg/m2). Majority of study participants were Sinhalese and according to the parity majority were primigravida pregnancies.
Majority of study participants were diagnosed as NVP Stage II (N=134:53.8%) by the PUQE scoring system. According to the clinical judgment Stage II of NVP was diagnosed among 127 (79.4%) study participants. Mean score of the PUQE scale was 9.08 (SD=2.35). Reliability assessment of the PUQE scoring system was reported as an excellent level (Cronbach alpha=0.804).
Five types of management strategies were detected among study participants. Significantly high percentage of study participants were given first line antiemetics and oral rehydration solutions. Significantly a smaller number of study participants were giver second-line oral antiemetics. Intravenous fluids were given in a significantly low percentage of participants. There were minimum number of study participants were given IVF-thiamine.
Calculated sensitivity of the PUQE scoring system to diagnose Stage I of NVP was 69.56% and calculated specificity was 96.35%. The positive predictive value was calculated as 76.19% and the negative predictive value of the PUQE scoring system was calculated as 94.96%. The likelihood ratio of the PUQE scoring system as a positive test was 19 (95% CI=7.74–47.0). The likelihood ratio of the PUQE scoring system as a negative test was 0.32 (95% CI=0017–0.59).
Calculated sensitivity of the PUQE scoring system to diagnose Stage II of NVP was 100% and calculated specificity was 78.78%. The positive predictive value was calculated as 94.77% and the negative predictive value of the PUQE scoring system was calculated as 100%. The likelihood ratio of the PUQE scoring system as a positive test was 4.71 (95% CI=2.40–8.50). The likelihood ratio of the PUQE scoring system as a negative test was zero.
All patients who were clinically detected as Stage II NVP patients were detected as Stage II NVP patients by the PUQE scoring system also. None of the false positives and false negatives regarding Stage II of NVP detected by the PUQE scoring system.
Comparison of study findings with other studies
Evaluation of nausea and vomiting in pregnancy using the PUQE and Nausea scale in Korea, Hyun Joung Choi provides some evidences that modified PUQE scoring system is a suitable tool to diagnose emesis status in the pregnancy. But some study participants did not resolve their hyperemesis status until the delivery in that study. They emphasize that the PUQE scoring system is more successful before the 20 weeks of gestation. In present study results also suggested that the PUQE scoring system is a more suitable tool for detecting emesis status. Almost all of the study participants in present study were in first trimester of the pregnancy. But the socio-demographic nature of the two study samples differed from each other. So, the comparison of every aspect of the scoring system does not provide highly valid information. There are several studies available in published databases but very rare studies were considered criterion validity of the PUQE scoring system. But the present study was analyzed in detail. So, the practical implicative importance is high in the present study findings because it provides information regarding different stages of hyperemesis in the pregnancy.
Implications of the study instrument in current gynecological practices in Sri Lanka
According to the PUQE scoring system, NVP status of the study participants can be practically used relevant to two stages. That is for NVP Stage I and Stage II. When Stage II of NVP is considered, predictability of the PUQE scoring system appears completely accurate. That is judgment of the PUQE scoring system always agree with clinical judgment. This can be considered as an extremely important information. Management strategies of a disease condition should be more accurately practical with increased severity of the disease because it can be developed to a high-risk status. At this status consuming more time may appear challenging to the healing process of the patient. According to the PUQE scoring system, when Stage II NVP state is judged, it is possible to make a direct and quick decision to admit the patient for necessary treatment. When considered according to the NVP Stage II, false negative state detection is not done by the PUQE scoring system.
Sensitivity of the test is 100% and it indicates that all the individuals with positive diseases are accurately identified by the PUQE tool. It helps to initiate the management strategies at the first instances. As PUQE identifies disease positives with extreme accuracy it is not necessary to obtain contributions from the clinical specialties for clinical judgment. It reduces treatment waiting time and it facilitates initiation of treatment with quick and moderate strategies. This prevents the progression of the patient’s condition up to Stage II, which appears to be a highly cost-effective prevention. On the other hand, for NVP Stage II, specificity of PUQE scoring system is less relevant to its sensitivity. That is production of false negative results is relatively less. With relevance to this study sample, it is at zero status, i.e., all the individuals with NVP Stage II are detected to be an extremely productive primary prevention opportunity. Due to this opportunity, an easy pathway is opened to prevent the progression of NVP up to the third stage. This creates a direct and indirect positive impact on the mental and physical well-being of the pregnant mothers and resource saving of the health care delivery system.
When NVP Stage I is considered predictive ability of PUQE scoring system, it is deviated towards the negative side. Its specificity and negative predictive values are higher than the sensitivity and the positive predictive value. Positive likelihood ratio of this appears to be in a higher state and negative likelihood ratio is at an intermediate state. It is identified that when applying the PUQE scoring system, a smaller number of false negatives are produced, i.e., there is a minimum probability of detecting patients in severe or moderate status as Stage I or mild clinical conditions. During these situations accurate and cost-effective usage of management strategies become higher.
This is extremely advantageous in a clinical morbidity, such as hyperemesis, which is not fatal but extremely uncomfortable. As mild state of NVP is accurately calculated, it is possible to initiative OPD-based treatment strategies following detection. On the other hand, application of this PUQE tool does not require highly skilled medical personnel. Therefore, it is possible to apply this tool to a pregnant mother through the public health midwife (PHM), who appears to be the closest health care personnel at domiciliary level. PUQE is a diagnostic tool strictly based on questions, a pregnant mother with a satisfactory education level can accurately assess her clinical condition by applying this tool herself. Therefore, it is possible to minimize hospitalization of pregnant mothers due to hyperemesis by publishing this tool.
LIMITATIONs
Range of main objectives of this study was extensive. Validation of the patient identification tool, classification and describing the patients, and describing the management strategies of the patients were done during this study. As a result, there were limitations of conducting the study and comprehensive application of statistical implementations. Especially, there are many practical difficulties of combining and executing a descriptive cross-sectional study design with a validation study which includes diagnostic accuracy.
As this study was conducted in a single study setting, observed management strategies appeared similar. This leads to reduction of the external validity of a descriptive analysis conducted on patient management strategies. External validity could have been increased if it was possible to use participants from different study settings while describing and comparing management strategies or if it was possible to use different clinical units in a single study setting.
On the other hand, hyperemesis is considered as a subjective measurement up to a certain extent. Apart from biophysiological conditions, severity of this parameter could differ with personality related conditions as well. Therefore, it is not possible to neglect the possibility of occurring a reliability error of study measurements. This can also be considered as an information bias of the study. During these conditions it is essential to increase the sample size in order to minimize the effect on the internal validity of the study. On the other hand heterogeneity of the study sample should also be increased. But it was not practical to fulfil both those requirements due to the time limitations which occurred while conducting as a postgraduate study. Therefore, the investigator had to satisfy with the minimum required sample size.
On the other hand, if it was possible to increase the number of incidents used for determining face validity and translation inconsistency validity of the acquired data could have been increased. This appeared to be a practical limitation in the present study design and attention should be paid on overcoming this limitation by using lager amount of resources in future studies.
Another confirmatory test was not used as a gold standard test in the diagnostic test accuracy component of the present study. Clinician judgment was considered as the confirmatory test. There was an opportunity of occurring a judgmental bias in this situation. Confirmatory test result could have been different, if it was possible to use clinical judgments of several clinicians. But according to the present study findings, it is obvious that judgments depict a clear inconsistency with the study tool. But the existing theoretical limitation was persistent throughout the study.
CONCLUSION
- First-line antiemetics and oral rehydration solutions are usually used for treating pregnancy-related nausea and vomiting.
- Stage II NVP conditions were detected with a higher prevalence when NVP status was diagnosed clinically and with PUQE scoring system.
- Specificity of PUQE scoring system appeared higher while detecting NVP Stage I and for NVP Stage II, sensitivity of PUQE scoring systems appeared high.
- For Stage III NVP, clinical judgments and PUQE scoring system values were completely compatible.
RECOMMENDATIONs
- Management strategies used for treating pregnancy-related hyperemesis status should be further studied. Conducting these studies at different study settings may help to achieve more successful results.
- PUQE tool can be used to diagnose NVP status more descriptively. Also, this PUQE tool is a valid tool which can be effectively used for patient management.
- Application of PUQE scoring system should be published by planning and implementing staff awareness programs for both curative sector and public health sector.
REFERENCES
1.
Einarson TR, Piwko C, Koren G. Quantifying the global rates of nausea and vomiting of pregnancy: A meta analysis. J Popul Ther Clin Pharmacol 2013;20(2):e171–83.
[Pubmed]

2.
Lacasse A, Lagoutte A, Ferreira E, Bérard A. Metoclopramide and diphenhydramine in the treatment of hyperemesis gravidarum: Effectiveness and predictors of rehospitalisation. Eur J Obstet Gynecol Reprod Biol 2009;143(1):43–9. [CrossRef]
[Pubmed]

3.
Gazmararian JA, Petersen R, Jamieson DJ, et al. Hospitalizations during pregnancy among managed care enrollees. Obstet Gynecol 2002;100(1):94–100. [CrossRef]
[Pubmed]

4.
Atanackovic G, Wolpin J, Koren G. Determinants of the need for hospital care among women with nausea and vomiting of pregnancy. Clin Invest Med 2001;24(2):90–3.
[Pubmed]

5.
Matthews A, Dowswell T, Haas DM, Doyle M, O’Mathúna DP. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev 2010;(9):CD007575. [CrossRef]
[Pubmed]

6.
Eliakim R, Abulafia O, Sherer DM. Hyperemesis gravidarum: A current review. Am J Perinatol 2000;17(4):207–18. [CrossRef]
[Pubmed]

7.
Miller F. Nausea and vomiting in pregnancy: The problem of perception – Is it really a disease? Am J Obstet Gynecol 2002;186(5 Suppl):S182–3. [CrossRef]
[Pubmed]

8.
Trogstad LIS, Stoltenberg C, Magnus P, Skjaerven R, Irgens LM. Recurrence risk in hyperemesis gravidarum. BJOG 2005;112(12):1641–5. [CrossRef]
[Pubmed]

9.
10.
Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. Am J Obstet Gynecol 2002;186(5 Suppl):S210–4. [CrossRef]
[Pubmed]

11.
O’Brien B, Relyea MJ. Use of indigenous explanations and remedies to further understand nausea and vomiting during pregnancy. Health Care Women Int 1999;20(1):49–61. [CrossRef]
[Pubmed]

12.
Woolhouse M. Complementary medicine for pregnancy complications. Aust Fam Physician 2006;35(9):695.
[Pubmed]

13.
Lacasse A, Rey E, Ferreira E, Morin C, Bérard A. Validity of a modified Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) scoring index to assess severity of nausea and vomiting of pregnancy. Am J Obstet Gynecol 2008;198(1):71.e1–7. [CrossRef]
[Pubmed]

14.
O’Brien B, Relyea MJ, Taerum T. Efficacy of P6 acupressure in the treatment of nausea and vomiting during pregnancy. Am J Obstet Gynecol 1996;174(2):708–15. [CrossRef]
[Pubmed]

15.
Zhou Q, O’Brien B, Soeken K. Rhodes Index of Nausea and Vomiting – Form 2 in pregnant women. A confirmatory factor analysis. Nurs Res 2001;50(4):251–7. [CrossRef]
[Pubmed]

17.
Kousen M. Treatment of nausea and vomiting in pregnancy. Am Fam Physician 1993;48(7):1279–84.
[Pubmed]

18.
Quinla JD, Hill DA. Nausea and vomiting of pregnancy. Am Fam Physician 2003;68(1):121–8.
[Pubmed]

19.
Swallow BL, Lindow SW, Masson EA, Hay DM. Development of an instrument to measure nausea and vomiting in pregnancy. J Obstet Gynaecol 2002;22(5):481–5. [CrossRef]
[Pubmed]

20.
Bsat FA, Hoffman DE, Seubert DE. Comparison of three outpatient regimens in the management of nausea and vomiting in pregnancy. J Perinatol 2003;23(7):531–5. [CrossRef]
[Pubmed]

21.
Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 Suppl):S198–203. [CrossRef]
[Pubmed]

23.
Rhodes VA, Watson PM, Johnson MH. Development of reliable and valid measures of nausea and vomiting. Cancer Nurs 1984;7(1):33–41.
[Pubmed]

24.
Mazzotta P, Stewart D, Atanackovic G, Koren G, Magee LA. Psychosocial morbidity among women with nausea and vomiting of pregnancy: Prevalence and association with anti-emetic therapy. J Psychosom Obstet Gynaecol 2000;21(3):129–36. [CrossRef]
[Pubmed]

25.
Koren G, Magee L, Attard C, et al. A novel method for the evaluation of the severity of nausea and vomiting of pregnancy. Eur J Obstet Gynecol Reprod Biol 2001;94(1):31–6. [CrossRef]
[Pubmed]

26.
Emelianova S, Mazzotta P, Einarson A, Koren G. Prevalence and severity of nausea and vomiting of pregnancy and effect of vitamin supplementation. Clin Invest Med 1999;22(3):106–10.
[Pubmed]

27.
Magee LA, Chandra K, Mazzotta P, Stewart D, Koren G, Guyatt GH. Development of a health-related quality of life instrument for nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 Suppl):S232–8. [CrossRef]
[Pubmed]

28.
Vikanes A, Grjibovski AM, Vangen S, Magnus P. Variations in prevalence of hyperemesis gravidarum by country of birth: A study of 900,074 pregnancies in Norway, 1967–2005. Scand J Public Health 2008;36(2):135–42. [CrossRef]
[Pubmed]

29.
Mazzotta P, Maltepe C, Navioz Y, Magee LA, Koren G. Attitudes, management and consequences of nausea and vomiting of pregnancy in the United States and Canada. Int J Gynaecol Obstet 2000;70(3):359–65. [CrossRef]
[Pubmed]

30.
Smith C, Crowther C, Beilby J, Dandeaux J. The impact of nausea and vomiting on women: A burden of early pregnancy. Aust N Z J Obstet Gynaecol 2000;40(4):397–401. [CrossRef]
[Pubmed]

31.
33.
Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. Br J Gen Pract 1993;43(371):245–8.
[Pubmed]

34.
O’Brien B, Naber S. Nausea and vomiting during pregnancy: Effects on the quality of women’s lives. Birth 1992;19(3):138–43. [CrossRef]
[Pubmed]

35.
Gadsby R, Barnie-Adshead AM. Nausea and Vomiting of Pregnancy: A Literature Review. Pregnancy Sickness Support; accessed 2008. Sections 7a, 9a. [Available at: https://pregnancysicknesssupport.org.uk].

37.
38.
39.
Neutel CI, Johansen HL. Measuring drug effectiveness by default: The case of Bendectin. Can J Public Health 1995;86(1):66–70.
[Pubmed]

40.
Arsenault MY, Lane CA, MacKinnon CJ, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can 2002;24(10):817–31.
[Pubmed]

41.
American College of Obstetrics and Gynecology. ACOG (American College of Obstetrics and Gynecology) Practice Bulletin: Nausea and vomiting of pregnancy. Obstet Gynecol 2004;103(4):803–14.
[Pubmed]

42.
Jewell D, Young G. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev 2003;(4):CD000145. [CrossRef]
[Pubmed]

43.
Mazzotta P, Magee LA. A risk-benefit assessment of pharmacological and nonpharmacological treatments for nausea and vomiting of pregnancy. Drugs 2000;59(4):781–800. [CrossRef]
[Pubmed]

44.
Debendox is not thalidomide. Lancet 1984;2(8396):205–6.
[Pubmed]

45.
Seto A, Einarson T, Koren G. Pregnancy outcome following first trimester exposure to antihistamines: Meta-analysis. Am J Perinatol 1997;14(3):119–24. [CrossRef]
[Pubmed]

46.
47.
Sahakian V, Rouse D, Sipes S, Rose N, Niebyl J. Vitamin B6 is effective therapy for nausea and vomiting of pregnancy: A randomized, double-blind placebo-controlled study. Obstet Gynecol 1991;78(1):33–6.
[Pubmed]

48.
Vutyavanich T, Wongtra-ngan S, Ruangsri R. Pyridoxine for nausea and vomiting of pregnancy: A randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol 1995;173(3 Pt 1):881–4. [CrossRef]
[Pubmed]

49.
Gadsby R, Barnie-Adshead AM. Nausea and Vomiting of Pregnancy: A Literature Review. Pregnancy Sickness Support; accessed 2008. Section 2e. [Available at: https://pregnancysicknesssupport.org.uk]

50.
Nelson MM, Forfar JO. Associations between drugs administered during pregnancy and congenital abnormalities of the fetus. Br Med J 1971;1(5748):523–7. [CrossRef]
[Pubmed]

51.
Nausea and Vomiting in pregnancy. [Available at: https://cks.nice.org.uk]

52.
Koren G, Levichek Z. The teratogenicity of drugs for nausea and vomiting of pregnancy: Perceived versus true risk. Am J Obstet Gynecol 2002;186(5 Suppl):S248–52. [CrossRef]
[Pubmed]

53.
Koren G, Maltepe C. Pre-emptive therapy for severe nausea and vomiting of pregnancy and hyperemesis gravidarum. J Obstet Gynaecol 2004;24(5):530–3. [CrossRef]
[Pubmed]

54.
55.
Verberg MFG, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11(5):527–39. [CrossRef]
[Pubmed]

56.
Källén B. Hyperemesis during pregnancy and delivery outcome: A registry study. Eur J Obstet Gynecol Reprod Biol 1987;26(4):291–302. [CrossRef]
[Pubmed]

57.
Zhang J, Cai WW. Severe vomiting during pregnancy: Antenatal correlates and fetal outcomes. Epidemiology 1991;2(6):454–7.
[Pubmed]

58.
Grjibovski AM, Vikanes A, Stoltenberg C, Magnus P. Consanguinity and the risk of hyperemesis gravidarum in Norway. Acta Obstet Gynecol Scand 2008;87(1):20–5. [CrossRef]
[Pubmed]

59.
Bailit JL. Hyperemesis gravidarium: Epidemiologic findings from a large cohort. Am J Obstet Gynecol 2005;193(3 Pt 1):811–4. [CrossRef]
[Pubmed]

60.
Dodds L, Fell DB, Joseph KS, Allen VM, Butler B. Outcomes of pregnancies complicated by hyperemesis gravidarum. Obstet Gynecol 2006;107(2 Pt 1):285–92. [CrossRef]
[Pubmed]

61.
Fell DB, Dodds L, Joseph KS, Allen VM, Butler B. Risk factors for hyperemesis gravidarum requiring hospital admission during pregnancy. Obstet Gynecol 2006;107(2 Pt 1):277–84. [CrossRef]
[Pubmed]

62.
Tan PC, Jacob R, Quek KF, Omar SZ. The fetal sex ratio and metabolic, biochemical, haematological and clinical indicators of severity of hyperemesis gravidarum. BJOG 2006;113(6):733–7. [CrossRef]
[Pubmed]

63.
Matsuo K, Ushioda N, Nagamatsu M, Kimura T. Hyperemesis gravidarum in Eastern Asian population. Gynecol Obstet Invest 2007;64(4):213–6. [CrossRef]
[Pubmed]

64.
Chiossi G, Neri I, Cavazzuti M, Basso G, Facchinetti F. Hyperemesis gravidarum complicated by Wernicke encephalopathy: Background, case report, and review of the literature. Obstet Gynecol Surv 2006;61(4):255–68. [CrossRef]
[Pubmed]

65.
Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol 1968;102(1):135–75. [CrossRef]
[Pubmed]

66.
Czaja JA. Food rejection by female rhesus monkeys during the menstrual cycle and early pregnancy. Physiol Behav 1975;14(5):579–87. [CrossRef]
[Pubmed]

67.
Hoskins J. How to manage the pregnant bitch. DVM News. 2003. [Available at: https://www.dvm360.com/view/how-manage-pregnant-bitch]

68.
Corey LA, Berg K, Solaas MH, Nance WE. The epidemiology of pregnancy complications and outcome in a Norwegian twin population. Obstet Gynecol 1992;80(6):989–94.
[Pubmed]

69.
Gadsby R, Barnie-Adshead AM, Jagger C. Pregnancy nausea related to women’s obstetric and personal histories. Gynecol Obstet Invest 1997;43(2):108–11. [CrossRef]
[Pubmed]

70.
Vellacott ID, Cooke EJ, James CE. Nausea and vomiting in early pregnancy. Int J Gynaecol Obstet 1988;27(1):57–62. [CrossRef]
[Pubmed]

71.
Erick M, Cox JT, Mogensen KM. ACOG practice bulletin 189: Nausea and vomiting of pregnancy. Obstet Gynecol 2018;131(5):935. [CrossRef]
[Pubmed]

72.
Matthews A, Haas DM, O’Mathúna DP, Dowswell T. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev 2015;2015(9):CD007575. [CrossRef]
[Pubmed]

73.
Lacasse A, Rey E, Ferreira E, Morin C, Bérard A. Nausea and vomiting of pregnancy: What about quality of life? BJOG 2008;115(12):1484–93. [CrossRef]
[Pubmed]

74.
Fejzo MS, Ingles SA, Wilson M, et al. High prevalence of severe nausea and vomiting of pregnancy and hyperemesis gravidarum among relatives of affected individuals. Eur J Obstet Gynecol Reprod Biol 2008;141(1):13–7. [CrossRef]
[Pubmed]

75.
Czeizel AE, Dudas I, Fritz G, Técsöi A, Hanck A, Kunovits G. The effect of periconceptional multivitamin-mineral supplementation on vertigo, nausea and vomiting in the first trimester of pregnancy. Arch Gynecol Obstet 1992;251(4):181–5. [CrossRef]
[Pubmed]

76.
Goodwin TM. Nausea and vomiting of pregnancy: An obstetric syndrome. Am J Obstet Gynecol 2002;186(5 Suppl):S184–9. [CrossRef]
[Pubmed]

77.
Bernstein L, Pike MC, Lobo RA, Depue RH, Ross RK, Henderson BE. Cigarette smoking in pregnancy results in marked decrease in maternal hCG and oestradiol levels. Br J Obstet Gynaecol 1989;96(1):92–6. [CrossRef]
[Pubmed]

78.
Weigel MM, Weigel RM. The association of reproductive history, demographic factors, and alcohol and tobacco consumption with the risk of developing nausea and vomiting in early pregnancy. Am J Epidemiol 1988;127(3):562–70. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Thilina Maduranga Liyanage - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthor declares no conflict of interest.
Copyright© 2024 Thilina Maduranga Liyanage. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.