|
Case Report
Understanding the behavior of a low-grade serous carcinoma
1 Fellow Gynecology-Oncologist, Department of Obstetrics & Gynecology, Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia
2 Trainee Lecturer, Department Obstetrics & Gynecology, Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia
3 Senior Lecturer/Consultant Gynecology-Oncologist, Department of Obstetrics & Gynecology, Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia
4 Clinical Lecturer, Department of Obstetrics & Gynaecology, Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia
Address correspondence to:
Lee Saw Joo
Hospital Universiti Sains Malaysia, Jln. Raja Perempuan Zainab 2, 16150 Kubang Kerian, Kelantan,
Malaysia
Message to Corresponding Author
Article ID: 100023G06LJ2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Joo LS, Ismail EHE, Ismail MP, Mahmood NMZN, Adnan WFW, Sung HP. Understanding the behavior of a low-grade serous carcinoma. Edorium J Gynecol Obstet 2019;5:100023G06LJ2019.ABSTRACT
Low-grade serous carcinoma (LGSC) of ovary represents 10% of all serous carcinoma of ovary and up to 7% of total ovarian malignancy. Lowgrade serous carcinoma being less common, hence have not been well studied. Here, we report a case of LGSC in a 22-year-old young woman in which her clinical presentation and her intraoperative findings were not in-line with her final histopathological diagnosis. Management of this patient has been challenging not only because of her young age, but also because LGSCs were found to be relatively chemoresistant as compared to its high-grade counterpart, however to date, there are no other more effective therapies with robust evidence. Nevertheless, reports on treatment with primary cytoreductive surgery followed by adjuvant hormonal therapy has shown promising results.
Keywords: Epithelial ovarian cancer, LGSC treatment option, Ovarian low-grade serous carcinoma, Type 1 and Type 2 epithelial ovarian carcinoma
INTRODUCTION
Serous adenocarcinoma of the ovary is the most common and lethal type of Epithelial Ovarian Carcinoma, comprising up to 68% [1]. Traditionally, ovarian serous carcinoma had been graded as—well, moderately and poorly differentiated, also known as Silverberg’s Grade [2]; based on the architecture (glandulary, papillary, or solid sheets), degree of nuclear atypia, and mitotic index. Recently, a two-tier system in which tumors are simply subdivided into low grade and high grade was introduced by Malpica et al. in 2004 [3]. Many studies done by various centers all over the world have shown that the two-tier system is easy to apply, reproducible, and based on underlying molecular biologic differences between low-grade serous carcinoma (LGSC) and highgrade serous carcinoma (HGSC) [3],[4],[5]. In these studies, apart from the differences at the molecular level and pathogenesis, LGSC and HGSC have clear differences in terms of epidemiology, clinical presentation, and treatment response as well as patients survival outcome.
Here, we report a case of LGSC of ovary which only represents 10% of all serous carcinoma of ovary [6]. Upon presentation, the huge tumor volume shows clinically and radiologically along with intraoperative findings of widely disseminated and infiltrative disease, one would think the patient would be having a carcinoma or sarcoma of highgrade type. The histopathological report comeback was beyond our clinical suspicion. Even though the advanced stage of presentation of her cancer can be attributed by the nature of a slow growing low-grade tumor, but the aggressive infiltrative disease found intraoperatively has made it worthwhile for our discussion. Furthermore, in view of her young age and the fact that LGSC is relatively chemoresistant, it was a challenge in treating her malignancy while trying to find a small possibility of preserving her fertility.
CASE REPORT
We have a 22-year-old Malay woman, a chemical engineering student, with no previous medical illness. She presented with progressively growing abdominal mass over two years duration. It was told to be initially confined to suprapubic region and gradually grew till occupying her whole abdomen. It had later caused her to have abdominal discomfort and urinary symptoms. She then began to have loss of appetite and weight about one month prior to presentation. Further questioning revealed history of on and off fever for the past three years, however denied abnormal per-vaginal discharge or high risk behavior. Infective disease workouts done previously in her university were all negative. She admitted taking alternative treatment supplement of a six-month course prior to presentation. Her menstrual and childhood history were unremarkable.
Patient was an obese woman with body mass index (BMI) 35 kg/m2 (Class II) with a normal vital signs, not septic. Cardiovascular, lung, breasts examinations were unremarkable. Her abdomen was grossly distended with a mass arising from pelvic region, extending toward xiphisternum mass was firm, tender, irregular, and fixed. Per rectal examination revealed extra-luminal mass felt anteriorly occupying whole Pouch of Douglas, fixed, and tender on pressure.
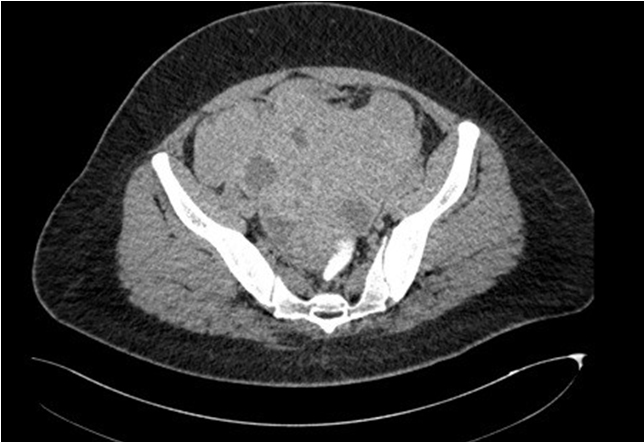
Trans-abdominal scan showed a huge right abdominopelvic mass, multiloculated measuring 20×15 cm, with mixed solid and cystic echogenicity with another smaller left sided mass of similar echogenicity. Uterus was not identified. Tumor markers showed only elevated CA 125 of 376 IU. Otherwise, other markers were within normal range. On computed tomography (CT) scan imaging (Figure 1), there was a huge heterogenously enhanced solid mass with cystic component in arising from the right side of pelvis measuring 13.3 cm ×16.4 cm ×11.9 cm extending extending toward the liver. In the pelvis, another smaller heterogenous enhancing solid cystic mass presents. The uterus was embedded within these mass and obliterated. These lesions have poor plane with each other and no clear plane with the uterus, bladder, and anterior abdominal wall. No radiological evidence of ascites is found. There were multiple subcentimeter para-aotic/paracaval lymphadenopathy. Mild right hydroureter, due to tumoral compression. Overall impression was in keeping with bilateral aggressive ovarian mass with local infiltration and nodal metastasis. No distant metastasis.
She was subjected to an exploratory laparotomy and debulking surgery. Intraoperatively, there was a huge omental cake upon entering the abdomen measuring 15×15 cm extending downward and adhered to the urinary bladder and uterus. The huge right ovarian tumor was solid-cystic sized 30×15 cm extending to right lobe of liver, with 800 cc of tumor necrotic pus content drained. Left side of pelvis was frozen pelvis with the left ovarian tumor densely infiltrated into the rectosigmoid colon posterior-laterally, and an enlarged uterus (equivalent of 16–18 weeks size gravid uterus) was seen infiltrating onto the urinary bladder anteriorly. There was also tumor seedling over the bowels which was excised. Only right salpingo-oophorectomy and omentectomy were done at that setting as a complete debulking surgery was not possible without causing morbidity to the bowels and urinary bladder. Liver and other solid organ were noted to be normal intraoperatively. Based on radiological and intraoperative findings, a clinical diagnosis of high grade bilateral ovarian tumor surgically International Federation of Gynecology and Obstetrics (FIGO) stage 3C with the differential diagnosis of possible carcinosarcoma had been made postoperatively.
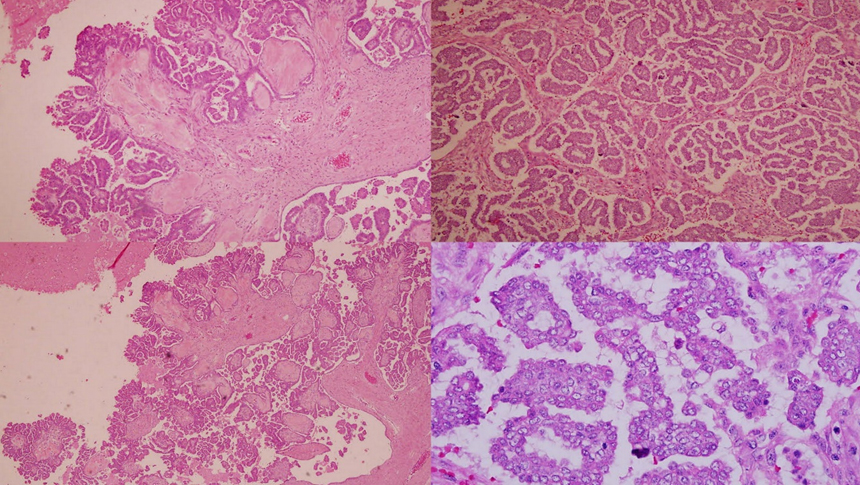
Histopathological report of right ovarian tumor and omental cake was suggestive of primary LGSC of ovary which showed malignant epithelial tumor forming hierarchical branching pattern characterized by irregular papillae and also micropapillae formations (Figure 2). The individual malignant cells exhibit mild to moderate nuclear pleomorphism, vesicular nuclei, prominent nucleoli and were also seen invading into the stroma. Mitoses was 7 in 10 high power field. Psammoma bodies are frequently seen. The surrounding stroma appears desmoplastic. Lymphovascular invasion was identified. And tumor seedling excised from bowel surface was positive for metastasis.
She was well postoperatively and was started on chemotherapy (three weekly paclitaxel/cisplatin regime). Patient showed poor response to chemotherapy with the CA 125 plateauing after 4th cycle of chemotherapy and the abdominopelvic mass increased in size as showed by her post-chemo CT imaging. Patient underwent second surgery whereby debulking anterior resection, hysterectomy, and left salpingo-oophorectomy were done. Bilateral ureteric stenting was done at the same setting to relieve the hydronephrosis. Postoperatively we tried to render the targeted therapy as adjuvant treatment however was deemed not suitable by the managing oncologist. Patient was left with option of expectant palliative care.
DISCUSSION
In recent years, the understanding of pathogenesis of epithelial ovarian carcinoma had underwent great evolution. Back in 1971, Fathalla [7],[8] proposed the theory of “incessant ovulation” being the mechanism of ovarian malignant transformation. However, there were doubts and questions raised on the poorly defined precursor lesion in this theory. In 1983, Cramer and Welch [8],[9] introduced the “gonadotropin theory,” postulating overstimulation of ovarian gonadotropin receptors by the gonadotropins (follicular stimulating hormone and luteinizing hormone) causing ovarian malignant transformation. This theory partly explained the higher incidence of ovarian malignancy in the postmenopausal age women and infertile women treated with gonadotropin.
Major evolution came after the discovery of BRCA gene mutation in 1995 by the scientists of National Institutes of Health, United States in patients with breast cancer. Further studies into BRCA 1 and 2 gene mutations had demonstrated the carrier of these gene mutations were not only at higher risk of getting breast cancer, they too have increased risks of having HGSC of ovaries, fallopian tubes, and peritoneal carcinomas [10],[11],[12]. However, BRCA gene mutations were not able to explain the occurrence of smaller percentage of patients with LGSC of ovaries which have been suggested to have a closer relationship with its borderline counterpart (Borderline Serous Ovarian Tumour) [13].
Type 1 and Type 2 ovarian tumorigenesis pathway was first proposed by Shih I and Kurman RJ in 2004 following extensive morphological and molecular genetic analysis [14],[15]. Based on this proposed model, all epithelial ovarian tumors were categorized into two main groups based on the two main pathways of tumorigenesis. Type 1 tumors are of the low grade neoplasm which arise in a stepwise manner from adenomas to borderline tumors to low-grade carcinomas. While Type 2 tumors are of high grade neoplasms which developed de novo without precursor lesion identified.
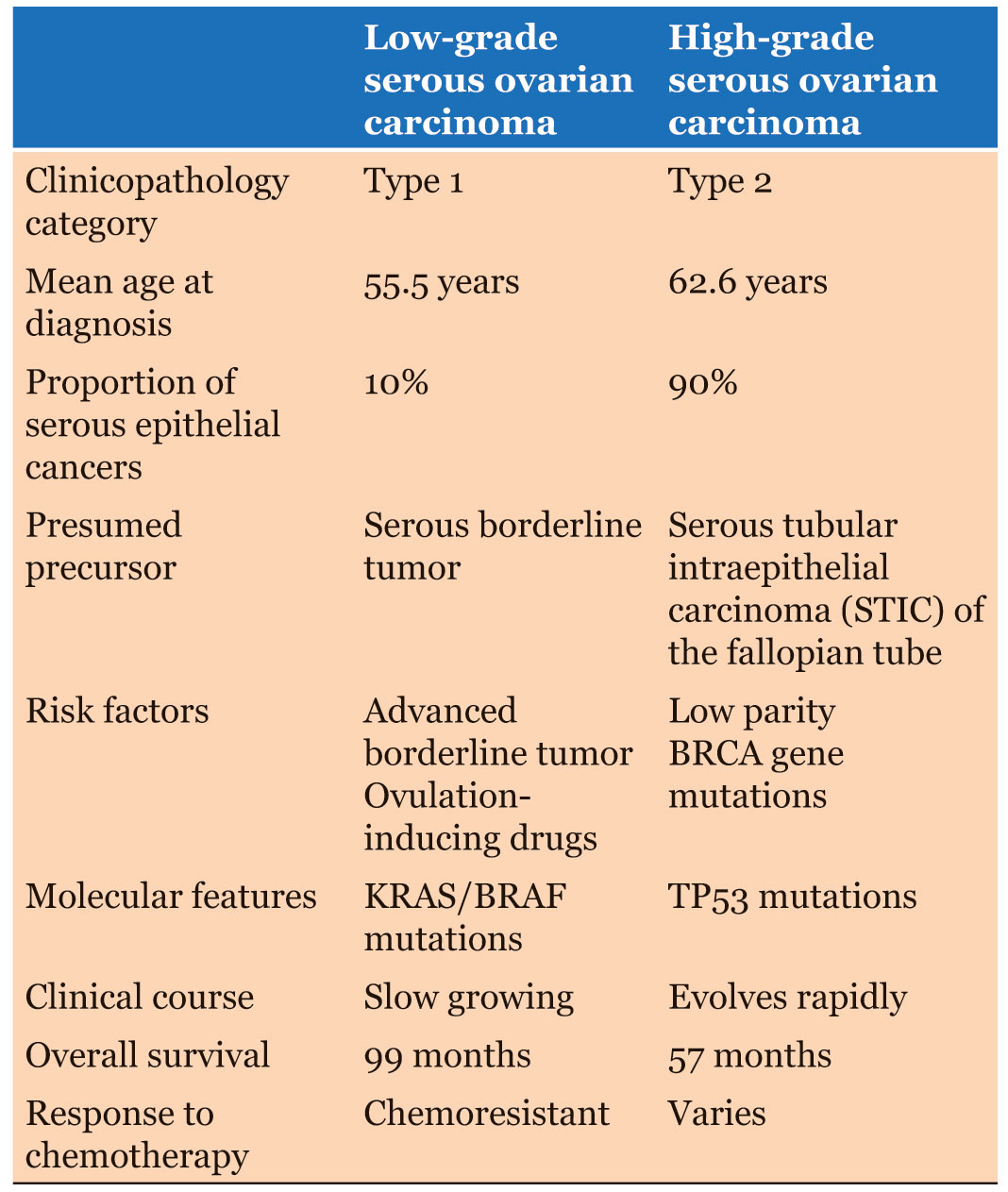
Generally, patients with Type 1 tumors (low grade serous, mucinous, endometrioid, malignant Brenner tumour, and clear cell carcinomas) usually demonstrate BRAF and KRAS molecular genetic mutations, take on slow clinical course, and have better prognosis. On the other hand, patients with Type 2 tumors (high grade serous, endometrioid, clear cell, undifferentiated, and carcinosarcomas) were shown to have frequent p53 genetic mutation, progress rapidly with poor outcome [15].
Narrowing down to LGSC, patients typically present at the earlier age (mean age of 55.5 years) as compared to patients with HGSC with the mean age of 62.6 years at presentation [16]. Our patient in particular is only 22 years of age. Despite having a slow growing clinical courses, patients with LGSC tend to present at advanced stage with prevalence reported as high as over 80% (Stage III/IV) [3], [17], just as like our patient. Nevertheless, overall survival rates of patients with LGSC were found to be significantly higher as compared to patients with HGSC; with 5 years and 10 years survival rate of 75 and 70% respectively in the LGSC group as compared to 40 and 26% respectively in the HGSC group [16]. Interestingly, one study pointed out that age at diagnosis of 35 and below, and presence of residual disease after primary treatment were predictors of poor survival and adverse outcome [18].
In regard to treatments for patients with LGSC, most centers advocate optimal debulking surgery followed by combined platinum/taxane-based adjuvant chemotherapy. This is despite the fact that LGSC was found to be relatively chemoresistant as compared to HGSC [19] (Table 1). Similar response was observed as well in cases of neoadjuvant chemotherapy and chemotherapy given in recurrent diseases [19]. It was postulated that the relative chemoresistant of the lowgrade cancer was attributed by the longer cell cycle [20]. One retrospective study published recently by Amanda N. Fader et al., patients with advanced stage LGSC treated with primary cytoreductive surgery followed by adjuvant hormonal therapy were compared to those treated with primary cytoreductive surgery followed by conventional adjuvant chemotherapy. The overall survival and two years progression-free in both group were almost comparable [21]. However, robust evidence with larger patient samples is required to support it as a standard management for LGSC.
In near future, treatment advancement may go toward targeted therapy on the specific somatic mutations (BRAF and KRAS mutations) which are commonly found in these tumors.
CONCLUSION
Management of LGSC remained to be a challenging one especially when patient presents at advanced stage. Survival prognosis of the patient is poor, it should be optimal debulking, and it cannot be achieved after primary surgery as LGSC tend to be resistant to chemotherapy. Optimal debulking on the contrary offers better survival outcome, however at the expense of patient’s fertility and surgical morbidities.
REFERENCES
1.
Soslow RA. Histologic subtypes of ovarian carcinoma: An overview. Int J Gynecol Pathol 2008;27(2):161–74.
[Pubmed]

2.
Silverberg SG. Histopathologic grading of ovarian carcinoma: A review and proposal. Int J Gynecol Pathol 2000;19(1):7–15.
[Pubmed]

3.
Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, Silva EG. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 2004;28(4):496–504.
[Pubmed]

4.
Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol 2009;16(5):267–82. [CrossRef]
[Pubmed]

5.
Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol 2006;108(2):361–8. [CrossRef]
[Pubmed]

6.
Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 2004;23(1):41–4. [CrossRef]
[Pubmed]

7.
Fathalla MF. Incessant ovulation: A factor in ovarian neoplasia? Lancet 1971;2(7716):163. [CrossRef]
[Pubmed]

8.
Aggarwal IM, Lim YH, Lim TYK. The fallopian tube as the origin of non-uterine pelvic high-grade serous carcinoma. The Obstetrician & Gynaecologist 2016;18:143–52. [CrossRef]

9.
Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71(4):717–21.
[Pubmed]

10.
Rubin SC, Blackwood MA, Bandera C, et al. BRCA1, BRCA2, and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: Relationship to family history and implications for genetic testing. Am J Obstet Gynecol. 1998;178(4):670–7. [CrossRef]
[Pubmed]

11.
Satagopan JM, Boyd J, Kauff ND, et al. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res 2002;8(12):3776–81.
[Pubmed]

12.
Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002;108(2):171–82. [CrossRef]
[Pubmed]

13.
Hogg R, Scurry J, Kim SN, Friedlander M, Hacker N. Microinvasion links ovarian serous borderline tumor and grade 1 invasive carcinoma. Gynecol Oncol 2007;106(1):44–51. [CrossRef]
[Pubmed]

14.
Cheng EJ, Kurman RJ, Wang M, et al. Molecular genetic analysis of ovarian serous cystadenomas. Lab Invest 2004;84(6):778–84. [CrossRef]
[Pubmed]

15.
Shih IeM, Kurman RJ. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am J Pathol 2004;164(5):1511–8.
[Pubmed]

16.
Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol 2008;198(4):459.e1–8. [CrossRef]
[Pubmed]

17.
Köbel M, Kalloger SE, Huntsman DG, et al. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol 2010;29(3):203–11. [CrossRef]
[Pubmed]

18.
Gershenson DM, Bodurka DC, Lu KH, et al. Impact of age and primary disease site on outcome in women with low-grade serous carcinoma of the ovary or peritoneum: Results of a large single-institution registry of a rare tumor. J Clin Oncol 2015;33(24):2675–82. [CrossRef]
[Pubmed]

19.
Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA. Low-grade serous ovarian cancer: A review. Gynecol Oncol 2016;143(2):433–8. [CrossRef]
[Pubmed]

20.
Schmeler KM, Gershenson DM. Low-grade serous ovarian cancer: A unique disease. Curr Oncol Rep 2008;10(6):519–23.
[Pubmed]

21.
Fader AN, Bergstrom J, Jernigan A, et al. Primary cytoreductive surgery and adjuvant hormonal monotherapy in women with advanced low-grade serous ovarian carcinoma: Reducing overtreatment without compromising survival? Gynecol Oncol 2017:147(1):85–91. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
The authors would like to thank Dr. Norzaliana Zawawi and Dr. Noorul Balqis Che Ibrahim of Pathology Department, HUSM for the histological images.
Author ContributionsLee Saw Joo - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Engku Husna Engku Ismail - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohd Pazudin Ismail - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nik Mohd Zaki Nik Mahmoo - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Wan Fadhlina Wan Adnan - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hoo Pek Sung - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Source of SupportNone
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Lee Saw Joo et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.