| Table of Contents |  |
|
Original Article
| ||||||
| Maternal serum adiponectin, leptin and adiponectin-leptin ratio as possible biomarkers of preeclampsia | ||||||
| Linda Ahenkorah Fondjo1, Enoch Appiah Adu Gyamfi2, William K. B. A. Owiredu1, Cornelius Archer Turpin3, Daniel Asante Mante4, Enoch Odame Anto2 | ||||||
|
1Lecturer, Molecular Medicine, KNUST, Kumasi, Ghana.
2Graduate Student, Molecular Medicine, KNUST, Kumasi, Ghana. 3Professor, Obstetric and Gyneacology, KNUST/SMS, Kumasi, Ghana. 4Medical Director, Obstetric and Gyneacology, Manhyia Government Hospital, Kumasi, Ghana. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Fondjo LA, Gyamfi EAA, Owiredu WKBA, Turpin CA, Mante DA, Anto EO. Maternal serum adiponectin, leptin and adiponectin-leptin ratio as possible biomarkers of preeclampsia. Edorium J Gynecol Obstet 2016;2:41–47. |
|
Abstract
|
|
Aims:
Preeclampsia is associated with significant maternal and perinatal morbidity and mortality, yet biomarkers for the condition have not been fully elucidated. As such, diagnosis of the condition in Ghana is based on blood pressure and urine protein measurement. The aim of this study was to compare the levels of adiponectin, leptin and the adiponectin-leptin ratio between normotensive pregnancy and preeclampsia.
Materials and Methods: 60 normotensive pregnant women and 60 preeclamptic women were recruited for this case-control study. Social and clinical information were obtained from all the participants. Maternal serum adiponectin and leptin were quantified using immunoassays. Differences in measured variables between groups were assessed with unpaired student's t-test; and associations among variables were described with Pearson's correlation coefficients. Results: Adiponectin levels (p=0.001), leptin levels (p=0.010) and the adiponectin-leptin ratio (p=0.001) were higher in preeclampsia than in normotensive pregnancy. There was a significant inverse correlation between adiponectin and leptin in the normotensive controls (r=-0.768, p=0.002) but this relationship was attenuated in preeclampsia (r=-0.290, p=0.052). Conclusion: Levels of adiponectin, leptin and the adiponectin-leptin ratio were elevated in the preeclamptic women and could serve as biomarkers for the diagnosis of preeclampsia. | |
|
Keywords:
Adiponectin, Adiponectin-leptin ratio, Biomarkers, Body mass index, Leptin, Preeclampsia
| |
|
Introduction
| ||||||
|
Preeclampsia is a pregnancy-specific syndrome characterized by hypertension and significant proteinuria. It affects 2–8% of pregnancies globally, and is associated with substantial maternal and fetal morbidity and mortality [1]. To date, the pathogenesis and pathophysiology of preeclampsia remains elusive, although several theories–comprising immunological, genetic, hematological and environmental factors–have been proposed. All these factors are believed to result in endothelial dysfunction, inflammation and angiogenesis, which are evident in preeclampsia [1] [2]. Since adiponectin and leptin – plasma proteins partly produced by the placenta [3] have been found to play roles in endothelial dysfunction, inflammation, angiogenesis and proteinuria, it has been hypothesized that these adipokines may play roles in the development of preeclampsia [1] [2]. If they are involved in the pathogenesis of preeclampsia, then their levels in preeclampsia and normotensive pregnancy may differ, thus serving as biomarkers and possible therapeutic targets for the diagnosis and treatment of preeclampsia respectively. Nonetheless, studies in this area have been inadequate and reports have been conflicting. While some studies have reported higher levels of adiponectin [4] and leptin [5] in preeclampsia, compared to normotensive pregnancy, others have reported differently [6] [7] [8]. Furthermore, not many of these studies have been conducted in Africa and for that matter Ghana. It is, therefore, evident that the levels of these markers- adiponectin, leptin and the adiponectin-leptin ratio in normotensive pregnancy and preeclampsia need additional investigation in the Ghanaian population. We hypothesize a priori that adiponectin, leptin and the adiponectin-leptin can serve as biomarkers for preeclampsia. This study thus aimed at comparing the levels of adiponectin, leptin and the adiponectin-leptin ratio between normotensive pregnancy and pregnancy complicated with preeclampsia. | ||||||
|
Materials and Methods
| ||||||
|
Study Site and Design Ethical Considerations Eligibility Criteria Study Population Anthropometric Measurement Blood Pressure Measurement Collection, Preservation and Biochemical Analysis of the Blood Samples Collection, Preservation and Biochemical Analysis of the Urine Samples Statistical Analysis | ||||||
|
Results | ||||||
|
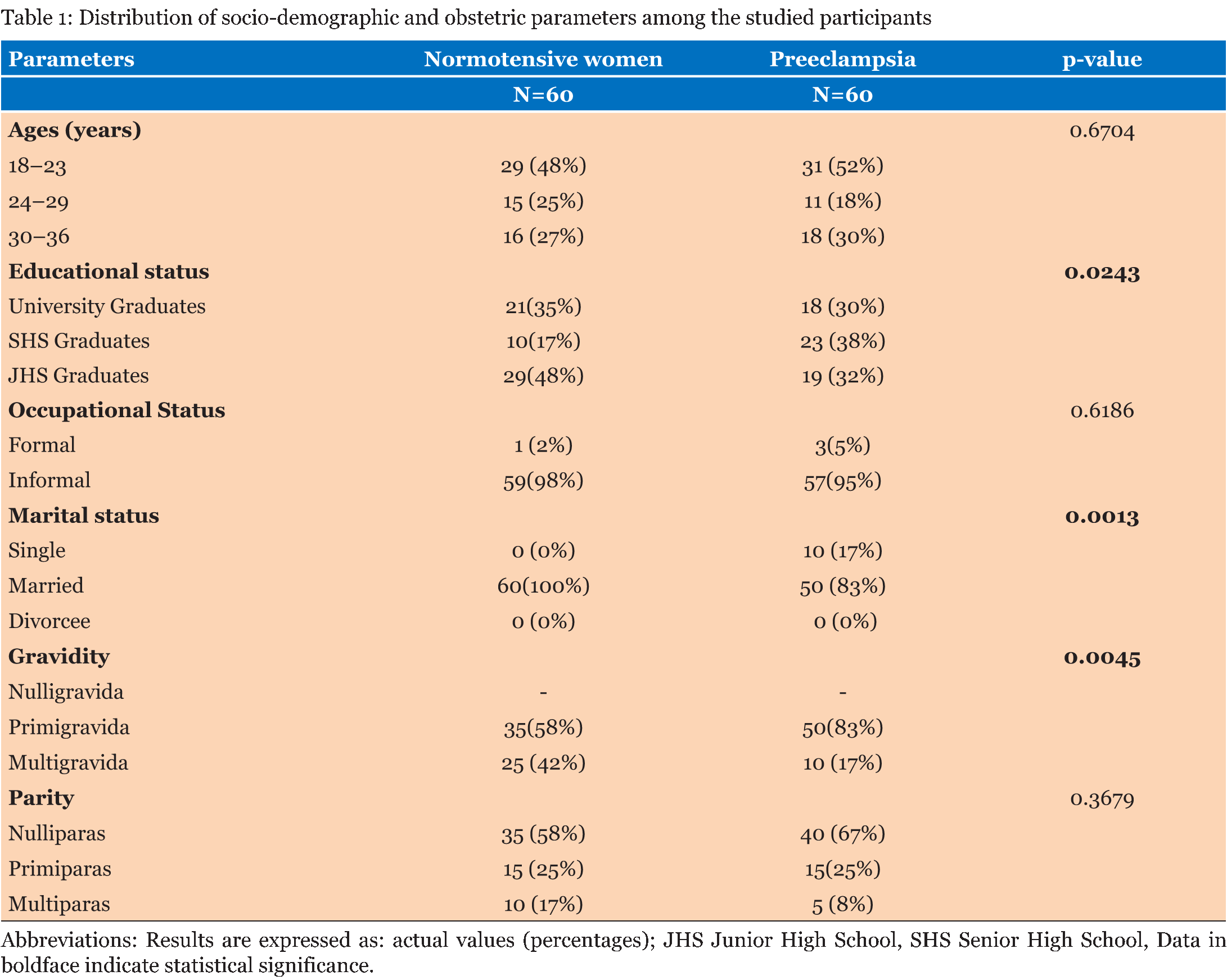
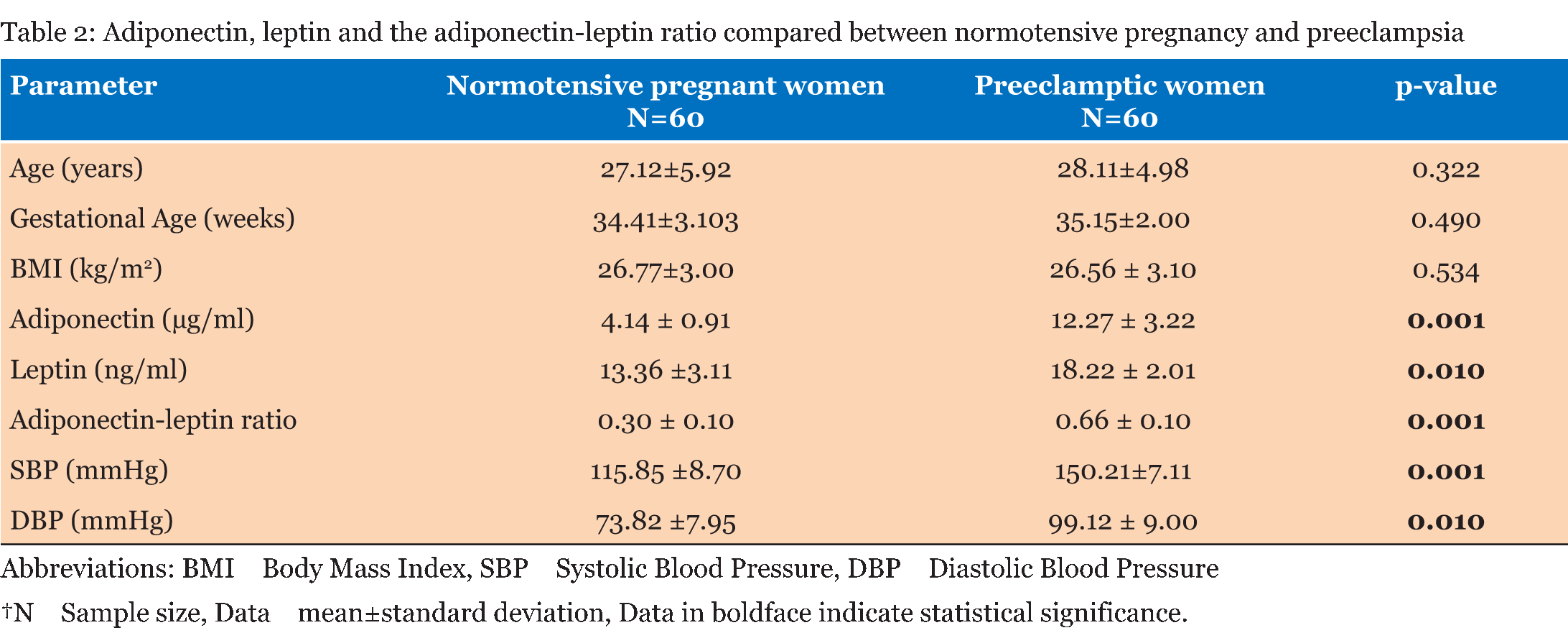
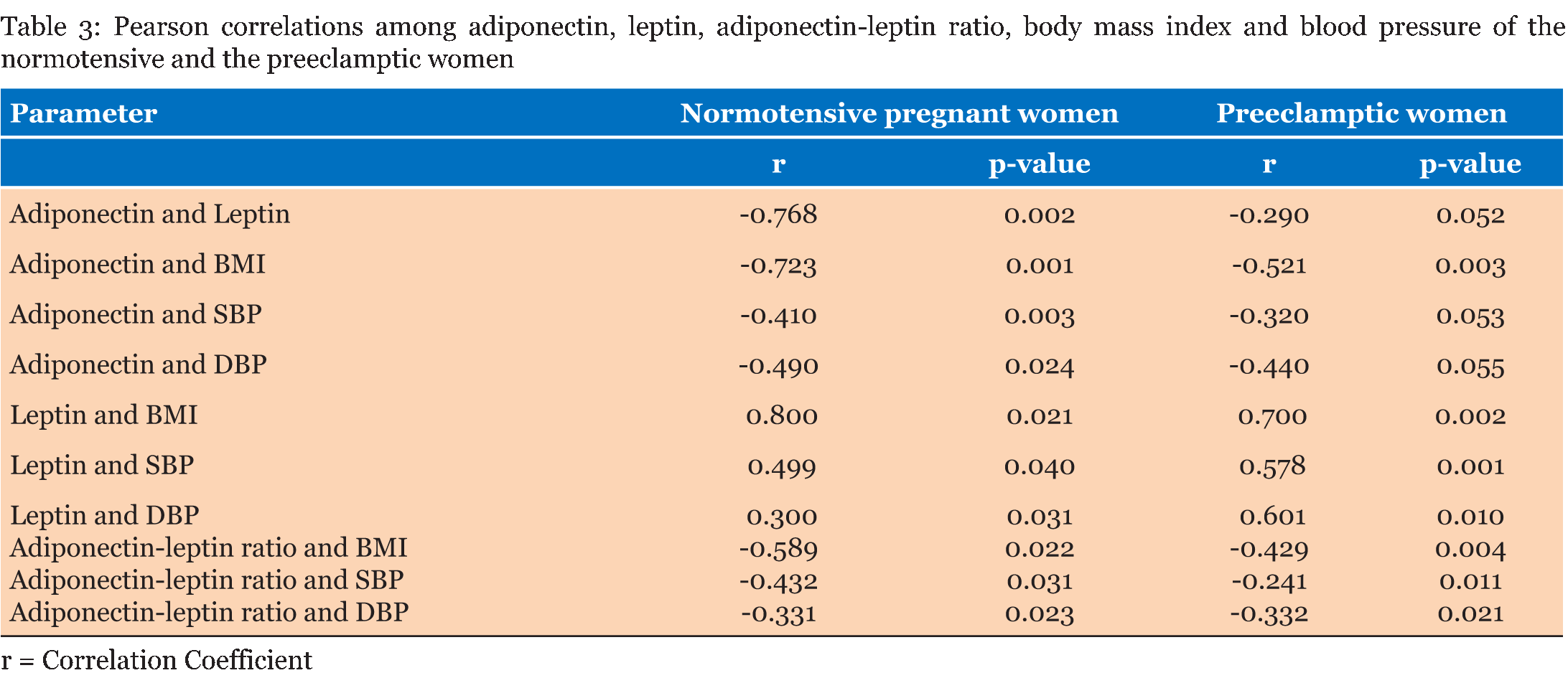
The entire study population comprised of 60 normotensive pregnant women and 60 women with preeclampsia. As given in Table 1, in both the normotensive pregnancy and preeclampsia most of the respondents were within the age range 18–23 years. All the participants had had formal education and there was significant between group differences in educational status. The majority of the participants were employed in the informal sector. None of the normotensive pregnant women were single, whereas 17% of the women with preeclampsia were unmarried, marital status also showed significant between group differences. While the prevalence of gravidity differed significantly between the normotensive and the preeclamptic women, the opposite held true for the prevalence of parity between the two groups. Although, most of the respondents, however, were nulliparas. Table 2 gives that the normotensive pregnant women and the preeclamptic women had comparable ages (p = 0.322), comparable gestational ages (p = 0.490) and comparable body mass indices (p = 0.534). As expected the preeclamptic women had higher systolic blood pressures (p=0.001) and diastolic blood pressures (p = 0.010). Adiponectin levels (p = 0.001), leptin levels (p = 0.010) and adiponectin-leptin ratios (p = 0.001) was higher in the women with preeclampsia as compared to the normotensive pregnant women. Table 3 gives the correlation among adiponectin, leptin and blood pressure in the normotensive pregnant women and the preeclamptic women. Among the normotensive pregnant women, adiponectin correlated inversely with leptin (p = 0.002), body mass index (p = 0.001), systolic blood pressure (p = 0.003) and diastolic blood pressure (p = 0.024); but all these relationships, except for body mass index, were blunted among the preeclamptic women (p > 0.05). Leptin correlated positively with body mass index (p = 0.021), systolic blood pressure (p = 0.040) and diastolic blood pressure (p = 0.031) among the normotensive pregnant women. Among the preeclamptic women, there was a strong linear relationship between leptin and systolic blood pressure (p = 0.001) and diastolic blood pressure (p = 0.010). Among the normotensive pregnant women, the adiponectin-leptin ratio correlated inversely with body mass index (p = 0.022), systolic blood pressure (p = 0.031) and diastolic blood pressure (p = 0.023). Among the preeclamptic women, similar inverse correlation were observed between the adiponectin-leptin ratio and body mass index (p = 0.004), systolic blood pressure (p = 0.011) and diastolic blood pressure (p = 0.021). | ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
It has been observed from this study that an inverse relationship exists between adiponectin and leptin in the normotensive pregnant women but not in the preeclamptic women. This therefore suggests that adiponectin and leptin levels are possibly altered by preeclampsia. The results of this study also indicate that adiponectin levels are significantly elevated in preeclampsia as compared to normotensive pregnancy, with the preeclamptic levels being approximately three times the normotensive pregnancy levels. Studies conducted by Abd-Alaleem et al. and Khosrowbeygi et al. are in consonance with the findings of this present study [4] [12]. However, studies conducted by authors contradict the findings of this study [6][7] [13]. While Hendler et al., [6] observed that the levels of adiponectin in normotensive pregnancy and preeclampsia are comparable, and Mazaki-Tovi et al. and Ouyang et al. observed significantly lower levels of adiponectin in preeclampsia than in normotensive pregnancy [7] [13]. Several reasons could account for the higher levels of adiponectin in preeclampsia –which is characterized by hypertension, proteinuria, inflammation and endothelial dysfunction [2] [14]. Overproduction of angiotensin II of the Renin-Angiotensin System (RAS) is linked to hypertension [15] and since it has been established that adiponectin antagonises the production of angiotensin II [16], it could be stated that hyperadiponectinaemia during preeclampsia is a possible feedback mechanism to antagonize angiotensin II production so as to control hypertension [17]. A study conducted by Sharma et al., [18] revealed that the expression of adiponectin receptor 1 (AdipoR1) in podocytes helps adiponectin to increase adenosine monophosphate kinase (AMPK) phosphorylation in podocytes to regulate kidney function against proteinuria. These investigators then concluded that adiponectin has a positive effect on the kidney, and that a fall in adiponectin levels may predispose to proteinuria, and a rise in adiponectin levels may be protective against proteinuria [18]. This indicates that elevation in the levels of adiponectin is, probably, part of the body's physiological mechanism that acts to forestall the development of proteinuria associated with preeclampsia. This assertion is supported by the findings of Hendler et al., [6] which indicated that alterations in renal function and an on-going adiponectin synthesis in adipose tissues might result in a rise in adiponectin levels. According to Tan et al., [19], adiponectin receptors are also expressed in human endothelial cells; so adiponectin binds to those receptors to regulate endothelial nitric oxide synthase (eNOS) activity which results in increased nitric oxide production in human aortic endothelial cells to offset endothelial dysfunction [20] . Since the endothelium plays a role in inflammation [21], protecting it from dysfunctional effects, will, in a way, control inflammatory processes. As posited by Robinson et al., [22], adiponectin exerts an anti-inflammatory effect through activation of all of its receptors by having direct actions on inflammatory cells and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and interacting with tumor necrosis factor alpha (TNF-α) [22]. This, therefore, indicates that hyperadiponectinaemia during preeclampsia is a step to restoring the integrity of the endothelium, and counteracting the inflammatory processes. All these positive feedback reactions of increasing adiponectin levels to annul the pathology, then possibly result in adiponectin resistance [23]. The above stated roles of adiponectin in preeclampsia depicts that a rise in the levels of adiponectin in preeclampsia, as observed in this study, could be suggestive of the body's physiological mechanism of antagonizing the pathophysiological progression of preeclampsia. Also, in this study, the levels of leptin were observed to be significantly higher in the preeclamptic women than in the normal pregnant women. The report of this study is in concordance with the reports of Mumtaz et al., [5], but in divergence with the reports of Martinez-Abundis et al. [24] and Laml et al. [8]. While Martinez-Abundis et al. [24] observed that serum leptin levels are comparable between normal pregnancy and preeclampsia, and so concluded that leptin cannot be used as a biomarker for preeclampsia, Laml et al. [8] observed decreased levels of leptin in preeclampsia. The positive correlation between leptin and blood pressure in the normal pregnant women, and the increase in strength of this positive correlation in preeclampsia suggests that the pathophysiological progression of preeclampsia may involve hyperleptinaemia. The finding that angiotensin II stimulates leptin production [25], and that higher leptin levels can cause hypertension [26], proteinuria and endothelial dysfunction [27], together with the finding that leptin plays a vital role during inflammation [28], may justify the higher levels of leptin seen in the preeclamptic women. Thus, unlike adiponectin, the rise of leptin levels during preeclampsia may not indicate a feedback mechanism to rectify the pathology but, rather, a pathophysiological mechanism to exacerbate the pathology. In this study, adiponectin-leptin ratio was two times higher in the preeclamptic women than in the normotensive pregnant women (100% increment). This suggest that during preeclampsia, the physiological mechanisms that underlie the secretion and circulation of adiponectin and leptin are altered, and that a transient increase in adiponectin-leptin ratio at any gestational age may be predictive of preeclampsia, and about 100% rise may indicate a preeclamptic state. | ||||||
|
Conclusion
| ||||||
|
The levels of adiponectin, leptin and the adiponectin-leptin ratio are elevated in preeclampsia. This indicates that they could serve as biomarkers for preeclampsia, and that determination of their levels could be integrated into the routine obstetric investigations that form the basis of antenatal care to help in early diagnosis of preeclampsia. | ||||||
|
Acknowledgements
| ||||||
|
The authors are thankful to the staff and clients of the Obstetric and Gyneacology Department of Manhyia Government Hospital, Kumasi Ghana. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Linda Ahenkorah Fondjo – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Enoch Appiah Adu Gyamfi – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published William K.B.A. Owiredu – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Cornelius Archer Turpin – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Daniel Asante Mante – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published Enoch Odame Anto – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Critical revision of the article, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Linda Ahenkorah Fondjo et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|