| Table of Contents |  |
|
Original Article
| ||||||
| Risk factors associated with diabetes mellitus among pregnant women in Hatyai Hospital, Thailand | ||||||
| Wannaro Prawit1, Rachatapantanakorn Benthira2, Sampurna Kakchapati3 | ||||||
|
1Doctor in Medicine, Medical Physician, Obstetrics and Gynecology Department, Hatyai hospital, Songkhla Thailand.
2Doctoral Degree in Research Methodology, Public Health Technical Officer, Health care systems development, Hatyai hospital, Songkhla Thailand. 3Post-Doctoral Public Health Scientist, Department of Mathematics and Computer Science, Prince of Songkla University, Muang, Pattani, Thailand. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Wannaro P, Rachatapantanakorn B, Kakchapati S. Risk factors associated with diabetes mellitus among pregnant women in Hatyai Hospital, Thailand. Edorium J Gynecol Obstet 2016;2:21–27. |
|
Abstract
|
|
Aims:
The aim of the study was to determine the factors associated with gestational diabetes mellitus (GDM) among pregnant women in Hatyai Hospital, Thailand.
Methods: Pregnant women with at least one clinical risk for GDM were screened by 50 g glucose challenge test (50 g GCT). If glucose level of pregnant women was 140 mg/dl or more, all positive screened women were tested by 100 g oral glucose tolerance test (100 g OGTT) to confirm GDM. Statistical analysis used chi-squared tests to assess statistically significant risk factors for GDM. Logistic regression models were used to identify the most important determinants associated with GDM. Results: About 588 pregnant women completed laboratory testing of GDM using 100 g OGTT and among them, 177 women (30.1%, 95%CI: 26.4–34.1) tested positive and diagnosis for GDM. After adjusting each factor for the confounding effects of other factors, age, previous history of GDM, weight gain, presence of hypertension were significantly associated with GDM in multivariate logistic analysis. GDM was higher among women aged 35 years and above, previous history of GDM, excessive weight gain and presence of hypertension. Conclusion: The high influencing risks for GDM were advanced maternal age, previous history of GDM, excessive weight gain and hypertension during pregnancy. Identifying diabetes early in pregnancy is vital for women to avoid health risk for them and their babies. | |
|
Keywords:
Gestation diabetes mellitus, Glucose challenge test, Logistic regression, Risk factor, Thailand
| |
|
Introduction
| ||||||
|
Diabetes mellitus (DM) is increasing globally and not exceptional in Thailand. The burden of diabetes is attributed to urbanization, changes in dietary patterns, decreasing levels of physical activity and increasing prevalence of obesity [1]. Gestational diabetes mellitus (GDM) is defined as glucose intolerance of varying degree with onset or first recognition during pregnancy. It is a common condition during pregnancy which is associated with negative short-term and long-term outcomes both for mothers and their babies. GDM have increased risk of preeclampsia during pregnancy, high risks for type 2 diabetes and cardiovascular diseases in their later life [2]. The babies born from GDM complicated pregnancy were risk for childhood obesity, impaired glucose tolerance and increased cardiovascular risk profile during adolescence and early adulthood [3] [4]. The latest WHO studies has also predicted the undoubtedly increase in the incidence of GDM especially in the developing countries due to the worldwide epidemic of glucose intolerance [5]. In the recent decades, Thailand has witnessed rapid lifestyle and socioeconomic changes with increasing westernization, characterized by changes in dietary intake and physical inactivity that have been linked in the rapidly increasing of diabetes in general population [6]. The most recent study among women aged 35 and over, family history of diabetes, previous birth over 4 kg, and known risk factors of GDM showed that the prevalence of GDM was 5.7%, and that of the 32 cases identified, 12 had one risk factor, one had two risk factors [7]. Prevalence of GDM varied by differences in screening practices (universal versus selective screening), population characteristics (e.g., average age and body mass index of pregnant women), testing method and diagnostic criteria [8] [9][10][11] [12]. Prevalence of GDM has been increasing over time, possibly related to increases in mean maternal age and weight [8] [9]. Studies identified that pregnant women with any of the following factors appeared to be at increased risk of developing GDM; A family history of diabetes, especially in first degree relatives [10], Pre-pregnancy weight ≥ 110% of ideal body weight or body mass index >30 kg/m2, significant weight gain in early adulthood and between pregnancies [11], or excessive gestational weight gain [9], age >25 years, previous delivery of a baby >9 pounds [4.1 kg] and history of impaired glucose tolerance [12]. The risk of GDM increases when multiple risk factors are present. Moreover, previous unexplained perinatal loss or birth of a malformed infant, Glycosuria at the first prenatal visit, polycystic ovary syndrome [12], Current use of glucocorticoids, essential hypertension or pregnancy-related hypertension and Metabolic syndrome were also associated with GDM. Women at risk of gestational diabetes were older (>25 years of age), with high BMI (>30 kg/m2), history of previous glucose intolerance or adverse pregnancy outcomes associated with gestational diabetes, and first degree relative with diabetes [13]. However, only 10% of the general obstetric population meets all of these criteria for low risk of developing GDM [14]. Intensive lifestyle interventions, such as greater physical activity and weight loss, are known to improve insulin sensitivity and insulin secretory function (and thereby reduce the risk of type 2 diabetes) but may not be the most appropriate advice at the start of pregnancy. Physical activity is likely an effective intervention for prevention and treatment of GDM, given its known effectiveness in prevention and treatment of type 2 diabetes [14]. The information on proportion of women suffering of GDM and factors associated with GDM are important to allow for rational planning and allocation of resources and the preventive strategies that may be undertaken in future. Identifying overt diabetes early in pregnancy may be important because these women are at increased risk of having a child with a congenital anomaly and may be at increased risk of complications from diabetes. Studies found that the threat of major congenital malformations is rise among women with GDM [15] [16]. Thus, the present study was, therefore, undertaken to assess the proportion of GDM among women attending Hatyai hospital and associated risk factors. These findings could assist policymakers for the prevention and control of GDM in Thailand. | ||||||
|
Materials and Methods
| ||||||
|
The pregnant woman who has at least one clinical risk for GDM were screened for GDM in antenatal care department of Hatyai Hospital from January 2014 to January 2015. The screened women were of gestational age of 24–28 weeks. About 602 pregnant women who had clinical risks for GDM were screened for GDM using 50 g GCT. Of these, 14 pregnant women had incomplete laboratory investigations and were excluded in the analysis. About 588 cases completed testing of GDM using 100 g OGTT. If glucose level of women was 140 mg/dl or more, all positive screened women were tested by 100 g oral glucose tolerance test (100 g OGTT) for confirmation of GDM. This criteria was recommended by NDDG (National Diabetes Data Group). Age, previous GDM, family history of diabetes mellitus, BMI, excessive weight gain, hypertension, presence of sugar in urine and size of fetus were selected as independent variables associated with GDM. The definite excessive weight gain was measure at 3 kg/month or more, increase BMI was measure 27 kg/m2 or more, presence of hypertension was measure at 140/90 mmHg or more, presence level of sugar in urine was measure at 1+ to 4+. Ethical approval for this study was obtained from the ethics committee of Hatyai Hospital. Statistical analyses were carried out using R program [17]. Bivariate analyses were conducted to estimate the association of demographic and behavioral variables with GDM among pregnant women using chi-squared tests. Logistic regression analyses were performed to determine variables associated with GDM proportion defined by combinations of the determinants, using the additive model: 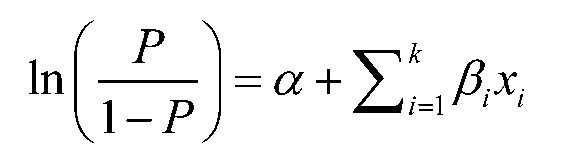
In this model, P is the expected probability of GDM; its intercepts though, are determinants and regression coefficients. Sum contrasts were used to obtain confidence intervals for comparing each proportion with the overall proportion. As it is necessary to construct specific contrasts for logistic regression, this can be accomplished by using weighted sum contrasts rather than treatment contrasts where the first level is left out from the model to be the reference [18]. The advantage of using appropriately weighted sum contrasts is that each proportion can be compared with the overall proportion rather than with a specified reference group. The computed 95% confidence intervals provide a way of classifying the levels of each factor into three groups according to whether each corresponding confidence interval exceeds, crosses or is below the overall proportion [19]. The confidence intervals compare percent of GDM in each category of a factor with the overall percent. For analysis, age was recoded into below 35 years and 35 years and above, BMI were recoded into less than 27 kg/m2 and 27 kg/m2 and more and size of fetus were recoded into below 4,000 grams and 4,000 grams and above. | ||||||
|
Results | ||||||
|
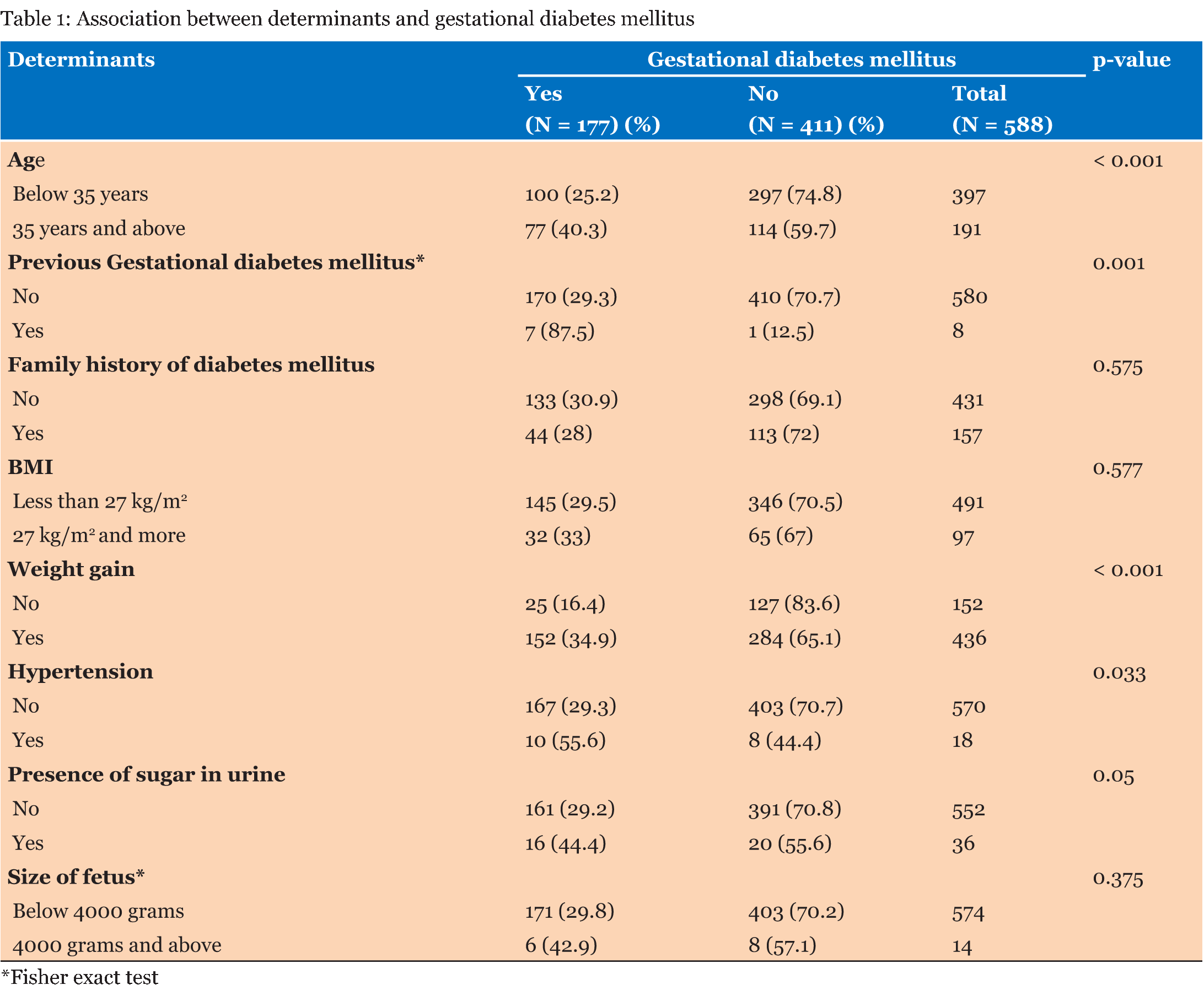
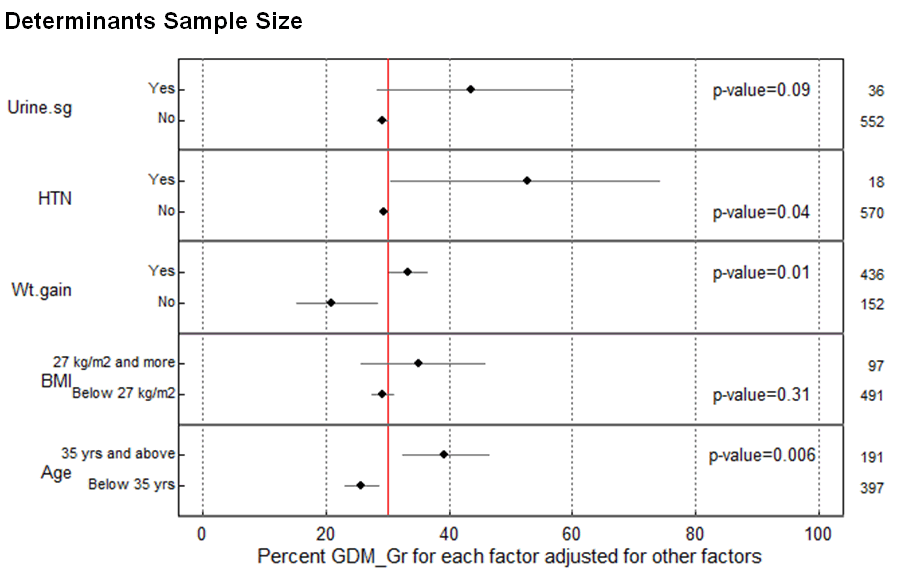
Among 588 women screened, 30.1% (95%CI: 26.4-34.1) tested positive for GDM. Table 1 examines the association between determinants and GDM. Age, previous history of GDM, excessive weight gain, hypertension and presence of sugar in urine were significantly associated with GDM (p-value <0.05). GDM was found to be associated with higher age groups; the proportion being 40% in the 35 years and above compared to younger age group, below 35 years (25%). Previous history of GDM was significantly associated GDM in the index pregnancy, however, the number of women with past history of GDM was small. GDM also varied by excessive weight gain; women who had gain weight excessive during pregnancy (35%) had higher GDM compared to those who had normal weight gain (29%). Hypertension also affected the GDM. More than half of women (56%) with hypertension had GDM. Presence of sugar in urine was also associated with GDM. Women who had urine in sugar had proportion of 44% compared to women who had no sugar in urine (29%). There were no statistical association between family history of diabetes mellitus, BMI and size of fetus with GDM (Table 1). Age, BMI, excessive weight gain in pregnancy, hypertension and presence of sugar in urine were included for multivariate logistic analysis. However, previous history of GDM and size of fetus were not included in logistic regression analysis because of small sample size. Figure 1 shows 95% confidence intervals of the proportion of GMD for each level of the determinants. This graph shows the corresponding adjusted percent with respect to the various levels of each of the determinants. In multivariate age, previous history of GDM, weight gain, hypertension were significantly associated with GDM (p-value <0.05). However, there was no significant association between BMI, presence of sugar in urine and GDM. There was increased in GDM with increasing age. The proportion of GDM among women aged 35 years and above was 38.1% (95%CI: 32.1–45.1) higher than below 35 years and below. Moreover, excessive weight gain during pregnancy was also associated with GDM, the proportion was about 33.9% (95%CI: 32.1–36.4), which is higher than women with normal weight gain (19.1%, 95%CI: 15.3–25.7). Presence of hypertension was also associated with GDM. The proportion of GDM among women with hypertension was 53.4% (95%CI: 30.5–76.3), which was higher than women with hypertension (29.3%, 95% CI: 29.1–29.5) (Figure 1). | ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
Gestational diabetes mellitus (GDM) continues to bear a substantial burden in pregnant women and their babies. This present study was undertaken to determine the proportion of pregnant women suffering from GDM and their associated risk factors in Hatyai hospital. This study showed a notable high proportion of GDM among pregnant women. The higher proportion may be due to inclusion of women who have at least one clinical risk for GDM. Moreover, two steps diagnosis of GDM were employed in the study, which was recommended by NDDG (National Diabetes Data Group). The study found that age, previous gestational diabetes mellitus, excessive weight gain and hypertension were significantly associated with GDM in multivariate analysis. No evidence on family history of diabetes mellitus, BMI and size of fetus with GDM was found in this study. Presence of sugar in urine was associated in bivariate analysis, however, it was not statistical significant in multivariate analysis. Consistent with other studies [8] [20], our study also showed that advanced maternal age was a strong risk factor for GDM. Maternal age is an established risk factor for GDM, the risk of GDM becomes significantly and progressively increased from 25 years onwards [20]. A significant association between history of GDM in previous pregnancy and development of GDM in the index pregnancy was seen, though the number of women with past history of GDM was small. Studies had found that there is high risk of development of GDM for women with prior GDM [10] [21]. Although family history of diabetes mellitus was a well-recognized risk factor for GDM, in our study no statistical association was found. Obesity was significantly associated with GDM [9] [11]. In our study, no significant associated between BMI and GDM was found. However, excessive weight gain during pregnancy was associated with GDM. Studies found that greater gestational weight gain in early pregnancy [9] [11], particularly during the first trimester, was associated with an increased risk of GDM. Women who develop GDM have higher gestational weight gain through 24 weeks. However, gestational excessive weight gain was a significant risk for GDM in the overweight or obese patient but not in patients who were underweight or had a normal BMI before pregnancy [9]. The rapid urbanization in Thailand in recent decades had brought about secular changes in lifestyles resulting in a rapid increase in the obesity and metabolic syndrome. While these risk factors, albeit highly modifiable, had contributed largely to the rising prevalence of diabetes in Thailand, the combination of these factors along with other risk factors such as advanced maternal age, overweight /obesity and increased blood pressure had also led to the rising prevalence of GDM in our population in the recent years. Given to the high risk for young-onset diabetes in women with GDM and the potential effects of GDM on cardiac metabolic risks in offspring born to mothers with GDM, the public, family and personal implications of this rising prevalence of GDM is particularly concerning. To prevent and control this public health emergency, large scale awareness, surveillance and prevention programs focusing on lifestyle modification before pregnancy and during early pregnancy in young women, notably those with risk factors, such as obesity and positive family history, need to be introduced to reduce the burden of GDM and to break the vicious cycle of diabetes. Tailoring of interventions based on the success of the Diabetes Prevention Program to women with gestational diabetes might alter maternal lifestyle behaviors, thereby improving their future post reproductive health profile. In addition, women may be responsive to implementing preventive interventions to reduce adiposity to improve their fetus health even more than their own. Pregnancy might provide an optimal time for targeted interventions for lifestyle modifications among women known to be at high risk for developing diabetes. | ||||||
|
Conclusion
| ||||||
|
The proportion of gestational diabetes mellitus (GDM) among women in this study was 30.1%. It was found out that the four factors such as increased age of pregnant women, previous gestational diabetes mellitus, excessive weight gain and hypertension predispose to GDM. This study also guides obstetricians and midwives in counseling and advising women of their risk of developing GDM during a given pregnancy based on individual risk factors. Moreover, it is, therefore, recommended for continuous surveillance by way of screening during pregnancy for purposes of early detection and prevention of developing gestational diabetes and comorbidity. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Prawit Wannaro – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Benthira Rachatapantanakorn – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Sampurna Kakchapati – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Prawit Wannaro et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|
|
About The Authors
| |||
| |||
| |||
| |||